Geert Jan Biessels
for the Heart-Brain Connection Consortium
An Interpretable Machine Learning Model with Deep Learning-based Imaging Biomarkers for Diagnosis of Alzheimer's Disease
Aug 15, 2023



Abstract:Machine learning methods have shown large potential for the automatic early diagnosis of Alzheimer's Disease (AD). However, some machine learning methods based on imaging data have poor interpretability because it is usually unclear how they make their decisions. Explainable Boosting Machines (EBMs) are interpretable machine learning models based on the statistical framework of generalized additive modeling, but have so far only been used for tabular data. Therefore, we propose a framework that combines the strength of EBM with high-dimensional imaging data using deep learning-based feature extraction. The proposed framework is interpretable because it provides the importance of each feature. We validated the proposed framework on the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset, achieving accuracy of 0.883 and area-under-the-curve (AUC) of 0.970 on AD and control classification. Furthermore, we validated the proposed framework on an external testing set, achieving accuracy of 0.778 and AUC of 0.887 on AD and subjective cognitive decline (SCD) classification. The proposed framework significantly outperformed an EBM model using volume biomarkers instead of deep learning-based features, as well as an end-to-end convolutional neural network (CNN) with optimized architecture.
Prior-knowledge-informed deep learning for lacune detection and quantification using multi-site brain MRI
Jun 18, 2023

Abstract:Lacunes of presumed vascular origin, also referred to as lacunar infarcts, are important to assess cerebral small vessel disease and cognitive diseases such as dementia. However, visual rating of lacunes from imaging data is challenging, time-consuming, and rater-dependent, owing to their small size, sparsity, and mimics. Whereas recent developments in automatic algorithms have shown to make the detection of lacunes faster while preserving sensitivity, they also showed a large number of false positives, which makes them impractical for use in clinical practice or large-scale studies. Here, we develop a novel framework that, in addition to lacune detection, outputs a categorical burden score. This score could provide a more practical estimate of lacune presence that simplifies and effectively accelerates the imaging assessment of lacunes. We hypothesize that the combination of detection and the categorical score makes the procedure less sensitive to noisy labels.
Cross-Cohort Generalizability of Deep and Conventional Machine Learning for MRI-based Diagnosis and Prediction of Alzheimer's Disease
Dec 16, 2020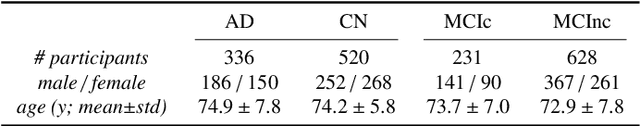
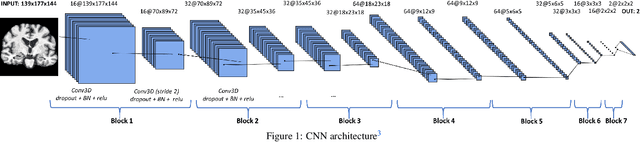
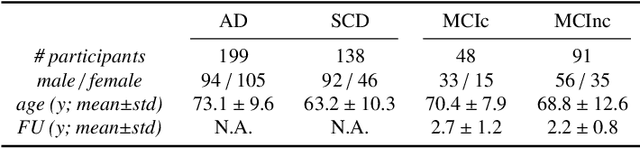
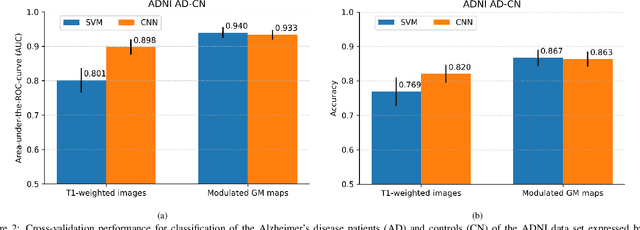
Abstract:This work validates the generalizability of MRI-based classification of Alzheimer's disease (AD) patients and controls (CN) to an external data set and to the task of prediction of conversion to AD in individuals with mild cognitive impairment (MCI). We used a conventional support vector machine (SVM) and a deep convolutional neural network (CNN) approach based on structural MRI scans that underwent either minimal pre-processing or more extensive pre-processing into modulated gray matter (GM) maps. Classifiers were optimized and evaluated using cross-validation in the ADNI (334 AD, 520 CN). Trained classifiers were subsequently applied to predict conversion to AD in ADNI MCI patients (231 converters, 628 non-converters) and in the independent Health-RI Parelsnoer data set. From this multi-center study representing a tertiary memory clinic population, we included 199 AD patients, 139 participants with subjective cognitive decline, 48 MCI patients converting to dementia, and 91 MCI patients who did not convert to dementia. AD-CN classification based on modulated GM maps resulted in a similar AUC for SVM (0.940) and CNN (0.933). Application to conversion prediction in MCI yielded significantly higher performance for SVM (0.756) than for CNN (0.742). In external validation, performance was slightly decreased. For AD-CN, it again gave similar AUCs for SVM (0.896) and CNN (0.876). For prediction in MCI, performances decreased for both SVM (0.665) and CNN (0.702). Both with SVM and CNN, classification based on modulated GM maps significantly outperformed classification based on minimally processed images. Deep and conventional classifiers performed equally well for AD classification and their performance decreased only slightly when applied to the external cohort. We expect that this work on external validation contributes towards translation of machine learning to clinical practice.
Standardized Assessment of Automatic Segmentation of White Matter Hyperintensities and Results of the WMH Segmentation Challenge
Apr 01, 2019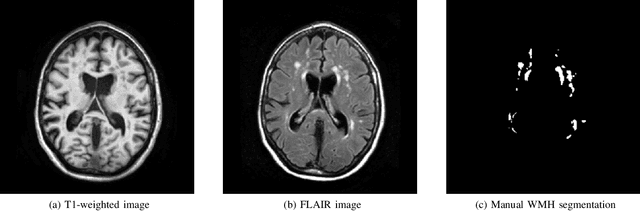
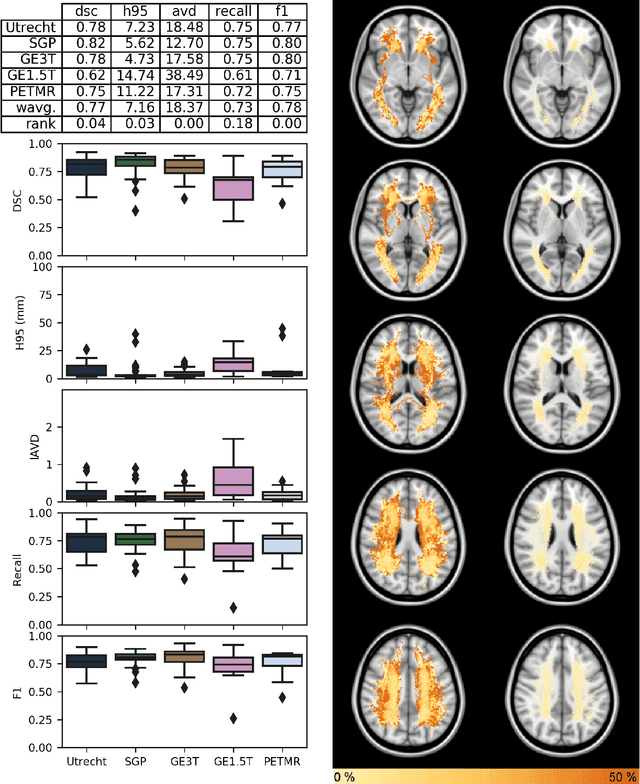
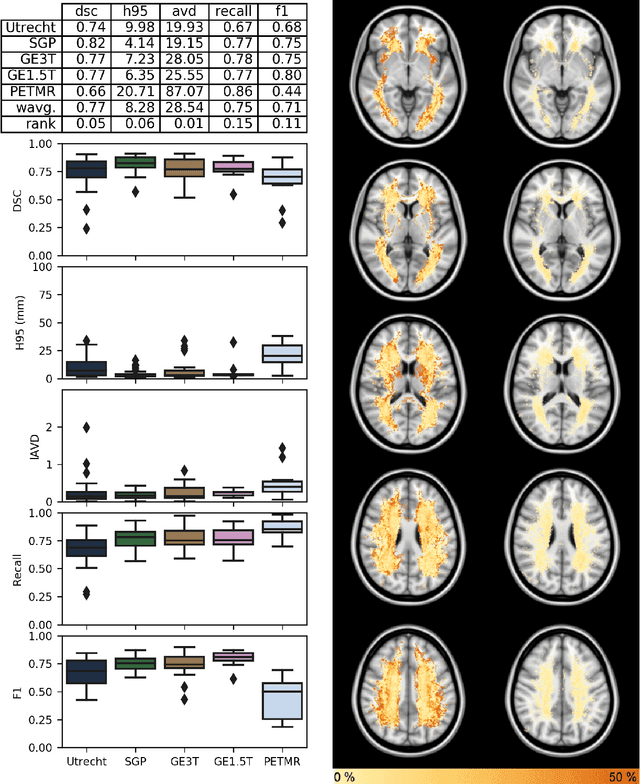
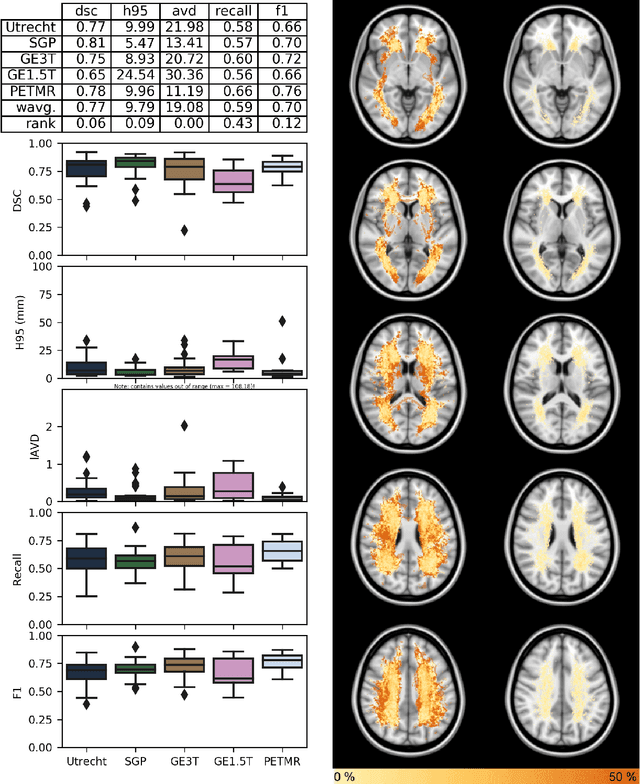
Abstract:Quantification of cerebral white matter hyperintensities (WMH) of presumed vascular origin is of key importance in many neurological research studies. Currently, measurements are often still obtained from manual segmentations on brain MR images, which is a laborious procedure. Automatic WMH segmentation methods exist, but a standardized comparison of the performance of such methods is lacking. We organized a scientific challenge, in which developers could evaluate their method on a standardized multi-center/-scanner image dataset, giving an objective comparison: the WMH Segmentation Challenge (https://wmh.isi.uu.nl/). Sixty T1+FLAIR images from three MR scanners were released with manual WMH segmentations for training. A test set of 110 images from five MR scanners was used for evaluation. Segmentation methods had to be containerized and submitted to the challenge organizers. Five evaluation metrics were used to rank the methods: (1) Dice similarity coefficient, (2) modified Hausdorff distance (95th percentile), (3) absolute log-transformed volume difference, (4) sensitivity for detecting individual lesions, and (5) F1-score for individual lesions. Additionally, methods were ranked on their inter-scanner robustness. Twenty participants submitted their method for evaluation. This paper provides a detailed analysis of the results. In brief, there is a cluster of four methods that rank significantly better than the other methods, with one clear winner. The inter-scanner robustness ranking shows that not all methods generalize to unseen scanners. The challenge remains open for future submissions and provides a public platform for method evaluation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge