Wenjie Kang
GL-ICNN: An End-To-End Interpretable Convolutional Neural Network for the Diagnosis and Prediction of Alzheimer's Disease
Jan 20, 2025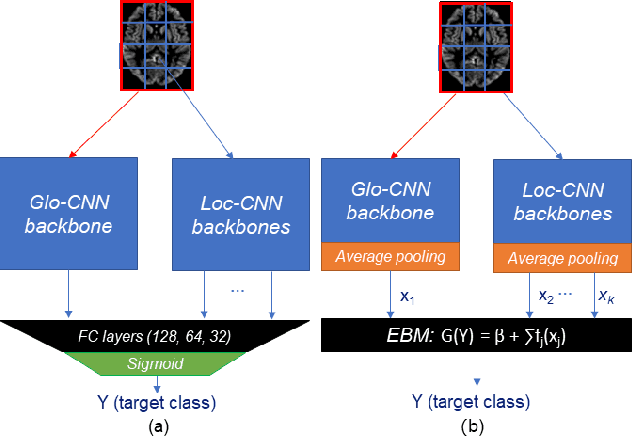
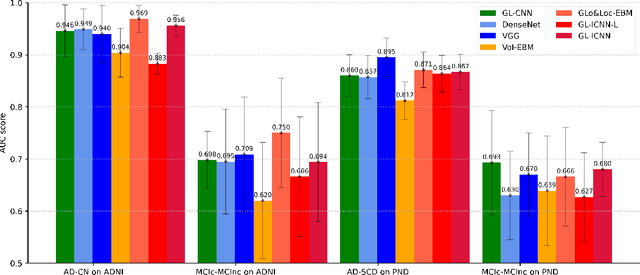
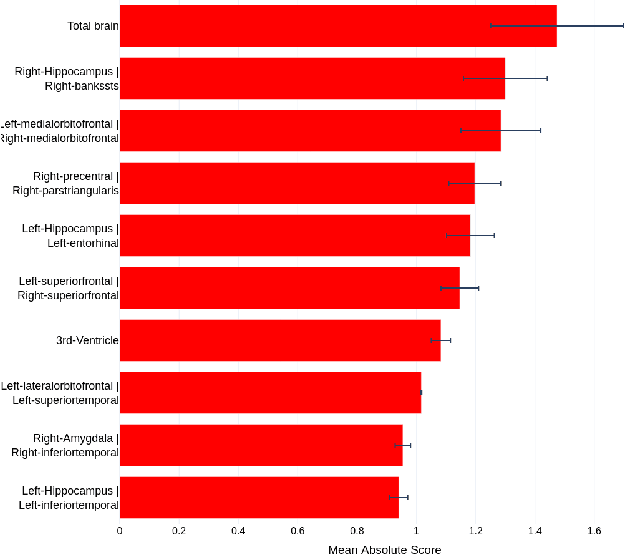
Abstract:Deep learning methods based on Convolutional Neural Networks (CNNs) have shown great potential to improve early and accurate diagnosis of Alzheimer's disease (AD) dementia based on imaging data. However, these methods have yet to be widely adopted in clinical practice, possibly due to the limited interpretability of deep learning models. The Explainable Boosting Machine (EBM) is a glass-box model but cannot learn features directly from input imaging data. In this study, we propose a novel interpretable model that combines CNNs and EBMs for the diagnosis and prediction of AD. We develop an innovative training strategy that alternatingly trains the CNN component as a feature extractor and the EBM component as the output block to form an end-to-end model. The model takes imaging data as input and provides both predictions and interpretable feature importance measures. We validated the proposed model on the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset and the Health-RI Parelsnoer Neurodegenerative Diseases Biobank (PND) as an external testing set. The proposed model achieved an area-under-the-curve (AUC) of 0.956 for AD and control classification, and 0.694 for the prediction of conversion of mild cognitive impairment (MCI) to AD on the ADNI cohort. The proposed model is a glass-box model that achieves a comparable performance with other state-of-the-art black-box models. Our code is publicly available at: https://anonymous.4open.science/r/GL-ICNN.
An Interpretable Machine Learning Model with Deep Learning-based Imaging Biomarkers for Diagnosis of Alzheimer's Disease
Aug 15, 2023



Abstract:Machine learning methods have shown large potential for the automatic early diagnosis of Alzheimer's Disease (AD). However, some machine learning methods based on imaging data have poor interpretability because it is usually unclear how they make their decisions. Explainable Boosting Machines (EBMs) are interpretable machine learning models based on the statistical framework of generalized additive modeling, but have so far only been used for tabular data. Therefore, we propose a framework that combines the strength of EBM with high-dimensional imaging data using deep learning-based feature extraction. The proposed framework is interpretable because it provides the importance of each feature. We validated the proposed framework on the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset, achieving accuracy of 0.883 and area-under-the-curve (AUC) of 0.970 on AD and control classification. Furthermore, we validated the proposed framework on an external testing set, achieving accuracy of 0.778 and AUC of 0.887 on AD and subjective cognitive decline (SCD) classification. The proposed framework significantly outperformed an EBM model using volume biomarkers instead of deep learning-based features, as well as an end-to-end convolutional neural network (CNN) with optimized architecture.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge