Wengong Jin
BindEnergyCraft: Casting Protein Structure Predictors as Energy-Based Models for Binder Design
May 27, 2025Abstract:Protein binder design has been transformed by hallucination-based methods that optimize structure prediction confidence metrics, such as the interface predicted TM-score (ipTM), via backpropagation. However, these metrics do not reflect the statistical likelihood of a binder-target complex under the learned distribution and yield sparse gradients for optimization. In this work, we propose a method to extract such likelihoods from structure predictors by reinterpreting their confidence outputs as an energy-based model (EBM). By leveraging the Joint Energy-based Modeling (JEM) framework, we introduce pTMEnergy, a statistical energy function derived from predicted inter-residue error distributions. We incorporate pTMEnergy into BindEnergyCraft (BECraft), a design pipeline that maintains the same optimization framework as BindCraft but replaces ipTM with our energy-based objective. BECraft outperforms BindCraft, RFDiffusion, and ESM3 across multiple challenging targets, achieving higher in silico binder success rates while reducing structural clashes. Furthermore, pTMEnergy establishes a new state-of-the-art in structure-based virtual screening tasks for miniprotein and RNA aptamer binders.
Reaction-conditioned De Novo Enzyme Design with GENzyme
Nov 10, 2024Abstract:The introduction of models like RFDiffusionAA, AlphaFold3, AlphaProteo, and Chai1 has revolutionized protein structure modeling and interaction prediction, primarily from a binding perspective, focusing on creating ideal lock-and-key models. However, these methods can fall short for enzyme-substrate interactions, where perfect binding models are rare, and induced fit states are more common. To address this, we shift to a functional perspective for enzyme design, where the enzyme function is defined by the reaction it catalyzes. Here, we introduce \textsc{GENzyme}, a \textit{de novo} enzyme design model that takes a catalytic reaction as input and generates the catalytic pocket, full enzyme structure, and enzyme-substrate binding complex. \textsc{GENzyme} is an end-to-end, three-staged model that integrates (1) a catalytic pocket generation and sequence co-design module, (2) a pocket inpainting and enzyme inverse folding module, and (3) a binding and screening module to optimize and predict enzyme-substrate complexes. The entire design process is driven by the catalytic reaction being targeted. This reaction-first approach allows for more accurate and biologically relevant enzyme design, potentially surpassing structure-based and binding-focused models in creating enzymes capable of catalyzing specific reactions. We provide \textsc{GENzyme} code at https://github.com/WillHua127/GENzyme.
Boltzmann-Aligned Inverse Folding Model as a Predictor of Mutational Effects on Protein-Protein Interactions
Oct 12, 2024



Abstract:Predicting the change in binding free energy ($\Delta \Delta G$) is crucial for understanding and modulating protein-protein interactions, which are critical in drug design. Due to the scarcity of experimental $\Delta \Delta G$ data, existing methods focus on pre-training, while neglecting the importance of alignment. In this work, we propose the Boltzmann Alignment technique to transfer knowledge from pre-trained inverse folding models to $\Delta \Delta G$ prediction. We begin by analyzing the thermodynamic definition of $\Delta \Delta G$ and introducing the Boltzmann distribution to connect energy with protein conformational distribution. However, the protein conformational distribution is intractable; therefore, we employ Bayes' theorem to circumvent direct estimation and instead utilize the log-likelihood provided by protein inverse folding models for $\Delta \Delta G$ estimation. Compared to previous inverse folding-based methods, our method explicitly accounts for the unbound state of protein complex in the $\Delta \Delta G$ thermodynamic cycle, introducing a physical inductive bias and achieving both supervised and unsupervised state-of-the-art (SoTA) performance. Experimental results on SKEMPI v2 indicate that our method achieves Spearman coefficients of 0.3201 (unsupervised) and 0.5134 (supervised), significantly surpassing the previously reported SoTA values of 0.2632 and 0.4324, respectively. Futhermore, we demonstrate the capability of our method on binding energy prediction, protein-protein docking and antibody optimization tasks.
RNAFlow: RNA Structure & Sequence Design via Inverse Folding-Based Flow Matching
May 29, 2024Abstract:The growing significance of RNA engineering in diverse biological applications has spurred interest in developing AI methods for structure-based RNA design. While diffusion models have excelled in protein design, adapting them for RNA presents new challenges due to RNA's conformational flexibility and the computational cost of fine-tuning large structure prediction models. To this end, we propose RNAFlow, a flow matching model for protein-conditioned RNA sequence-structure design. Its denoising network integrates an RNA inverse folding model and a pre-trained RosettaFold2NA network for generation of RNA sequences and structures. The integration of inverse folding in the structure denoising process allows us to simplify training by fixing the structure prediction network. We further enhance the inverse folding model by conditioning it on inferred conformational ensembles to model dynamic RNA conformations. Evaluation on protein-conditioned RNA structure and sequence generation tasks demonstrates RNAFlow's advantage over existing RNA design methods.
Generative Enzyme Design Guided by Functionally Important Sites and Small-Molecule Substrates
May 13, 2024Abstract:Enzymes are genetically encoded biocatalysts capable of accelerating chemical reactions. How can we automatically design functional enzymes? In this paper, we propose EnzyGen, an approach to learn a unified model to design enzymes across all functional families. Our key idea is to generate an enzyme's amino acid sequence and their three-dimensional (3D) coordinates based on functionally important sites and substrates corresponding to a desired catalytic function. These sites are automatically mined from enzyme databases. EnzyGen consists of a novel interleaving network of attention and neighborhood equivariant layers, which captures both long-range correlation in an entire protein sequence and local influence from nearest amino acids in 3D space. To learn the generative model, we devise a joint training objective, including a sequence generation loss, a position prediction loss and an enzyme-substrate interaction loss. We further construct EnzyBench, a dataset with 3157 enzyme families, covering all available enzymes within the protein data bank (PDB). Experimental results show that our EnzyGen consistently achieves the best performance across all 323 testing families, surpassing the best baseline by 10.79% in terms of substrate binding affinity. These findings demonstrate EnzyGen's superior capability in designing well-folded and effective enzymes binding to specific substrates with high affinities.
SurfPro: Functional Protein Design Based on Continuous Surface
May 07, 2024Abstract:How can we design proteins with desired functions? We are motivated by a chemical intuition that both geometric structure and biochemical properties are critical to a protein's function. In this paper, we propose SurfPro, a new method to generate functional proteins given a desired surface and its associated biochemical properties. SurfPro comprises a hierarchical encoder that progressively models the geometric shape and biochemical features of a protein surface, and an autoregressive decoder to produce an amino acid sequence. We evaluate SurfPro on a standard inverse folding benchmark CATH 4.2 and two functional protein design tasks: protein binder design and enzyme design. Our SurfPro consistently surpasses previous state-of-the-art inverse folding methods, achieving a recovery rate of 57.78% on CATH 4.2 and higher success rates in terms of protein-protein binding and enzyme-substrate interaction scores.
Unsupervised Protein-Ligand Binding Energy Prediction via Neural Euler's Rotation Equation
Jan 25, 2023



Abstract:Protein-ligand binding prediction is a fundamental problem in AI-driven drug discovery. Prior work focused on supervised learning methods using a large set of binding affinity data for small molecules, but it is hard to apply the same strategy to other drug classes like antibodies as labelled data is limited. In this paper, we explore unsupervised approaches and reformulate binding energy prediction as a generative modeling task. Specifically, we train an energy-based model on a set of unlabelled protein-ligand complexes using SE(3) denoising score matching and interpret its log-likelihood as binding affinity. Our key contribution is a new equivariant rotation prediction network called Neural Euler's Rotation Equations (NERE) for SE(3) score matching. It predicts a rotation by modeling the force and torque between protein and ligand atoms, where the force is defined as the gradient of an energy function with respect to atom coordinates. We evaluate NERE on protein-ligand and antibody-antigen binding affinity prediction benchmarks. Our model outperforms all unsupervised baselines (physics-based and statistical potentials) and matches supervised learning methods in the antibody case.
Antibody-Antigen Docking and Design via Hierarchical Equivariant Refinement
Jul 14, 2022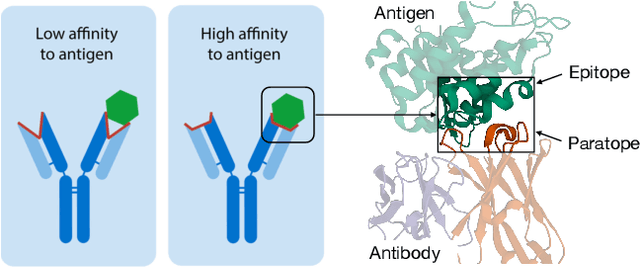
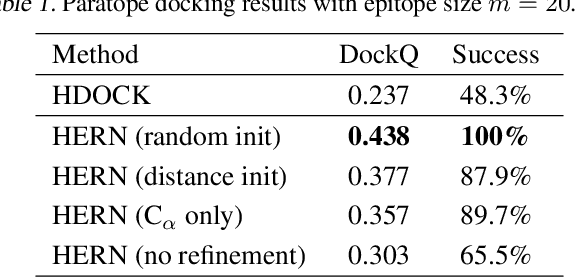

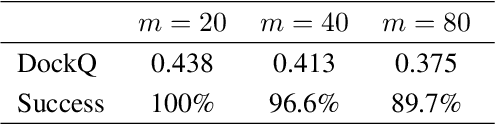
Abstract:Computational antibody design seeks to automatically create an antibody that binds to an antigen. The binding affinity is governed by the 3D binding interface where antibody residues (paratope) closely interact with antigen residues (epitope). Thus, predicting 3D paratope-epitope complex (docking) is the key to finding the best paratope. In this paper, we propose a new model called Hierarchical Equivariant Refinement Network (HERN) for paratope docking and design. During docking, HERN employs a hierarchical message passing network to predict atomic forces and use them to refine a binding complex in an iterative, equivariant manner. During generation, its autoregressive decoder progressively docks generated paratopes and builds a geometric representation of the binding interface to guide the next residue choice. Our results show that HERN significantly outperforms prior state-of-the-art on paratope docking and design benchmarks.
Iterative Refinement Graph Neural Network for Antibody Sequence-Structure Co-design
Oct 15, 2021
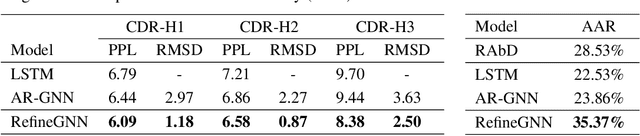
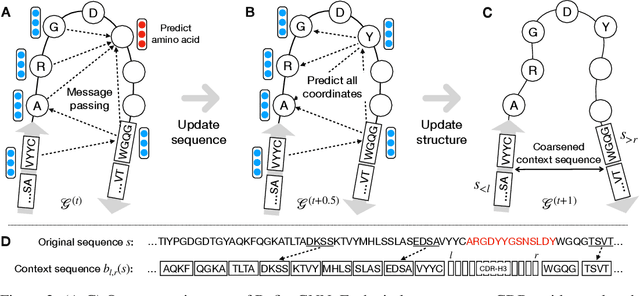
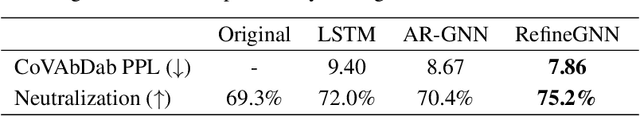
Abstract:Antibodies are versatile proteins that bind to pathogens like viruses and stimulate the adaptive immune system. The specificity of antibody binding is determined by complementarity-determining regions (CDRs) at the tips of these Y-shaped proteins. In this paper, we propose a generative model to automatically design the CDRs of antibodies with enhanced binding specificity or neutralization capabilities. Previous generative approaches formulate protein design as a structure-conditioned sequence generation task, assuming the desired 3D structure is given a priori. In contrast, we propose to co-design the sequence and 3D structure of CDRs as graphs. Our model unravels a sequence autoregressively while iteratively refining its predicted global structure. The inferred structure in turn guides subsequent residue choices. For efficiency, we model the conditional dependence between residues inside and outside of a CDR in a coarse-grained manner. Our method achieves superior log-likelihood on the test set and outperforms previous baselines in designing antibodies capable of neutralizing the SARS-CoV-2 virus.
Modeling Drug Combinations based on Molecular Structures and Biological Targets
Nov 09, 2020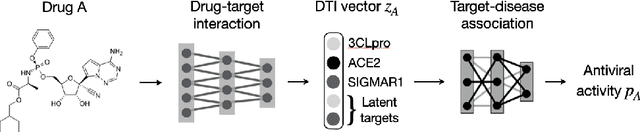
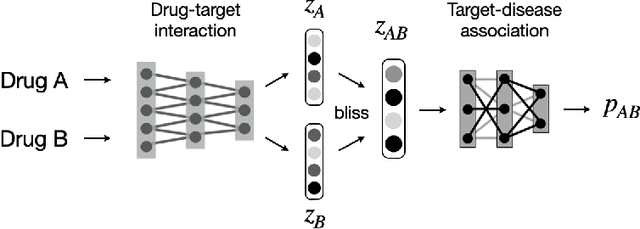
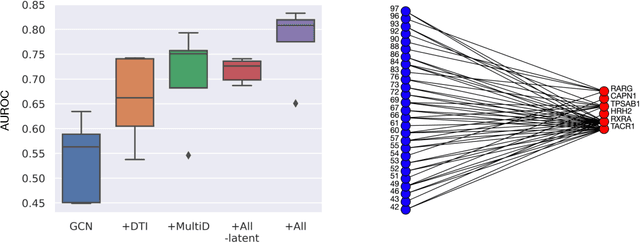
Abstract:Drug combinations play an important role in therapeutics due to its better efficacy and reduced toxicity. Since validating drug combinations via direct screening is prohibitively expensive due to combinatorial explosion, recent approaches have applied machine learning to identify synergistic combinations for cancer. However, these approaches is not readily applicable to many diseases with limited combination data. Motivated by the fact that drug synergy is closely tied with biological targets, we propose a model that jointly learns drug-target interaction and drug synergy. The model, parametrized as a graph convolutional network, consists of two parts: a drug-target interaction and target-disease association module. These modules are trained together on drug combination screen as well as abundant drug-target interaction data. Our model is trained and evaluated on two SARS-CoV-2 drug combination screens and achieves 0.777 test AUC, which is 10% higher than the model trained without drug-target interaction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge