Mengyu Wang
LLM Review: Enhancing Creative Writing via Blind Peer Review Feedback
Jan 12, 2026Abstract:Large Language Models (LLMs) often struggle with creative generation, and multi-agent frameworks that improve reasoning through interaction can paradoxically hinder creativity by inducing content homogenization. We introduce LLM Review, a peer-review-inspired framework implementing Blind Peer Review: agents exchange targeted feedback while revising independently, preserving divergent creative trajectories. To enable rigorous evaluation, we propose SciFi-100, a science fiction writing dataset with a unified framework combining LLM-as-a-judge scoring, human annotation, and rule-based novelty metrics. Experiments demonstrate that LLM Review consistently outperforms multi-agent baselines, and smaller models with our framework can surpass larger single-agent models, suggesting interaction structure may substitute for model scale.
Grading Scale Impact on LLM-as-a-Judge: Human-LLM Alignment Is Highest on 0-5 Grading Scale
Jan 06, 2026Abstract:Large language models (LLMs) are increasingly used as automated evaluators, yet prior works demonstrate that these LLM judges often lack consistency in scoring when the prompt is altered. However, the effect of the grading scale itself remains underexplored. We study the LLM-as-a-judge problem by comparing two kinds of raters: humans and LLMs. We collect ratings from both groups on three scales and across six benchmarks that include objective, open-ended subjective, and mixed tasks. Using intraclass correlation coefficients (ICC) to measure absolute agreement, we find that LLM judgments are not perfectly consistent across scales on subjective benchmarks, and that the choice of scale substantially shifts human-LLM agreement, even when within-group panel reliability is high. Aggregated over tasks, the grading scale of 0-5 yields the strongest human-LLM alignment. We further demonstrate that pooled reliability can mask benchmark heterogeneity and reveal systematic subgroup differences in alignment across gender groups, strengthening the importance of scale design and sub-level diagnostics as essential components of LLM-as-a-judge protocols.
Memorization in 3D Shape Generation: An Empirical Study
Dec 29, 2025Abstract:Generative models are increasingly used in 3D vision to synthesize novel shapes, yet it remains unclear whether their generation relies on memorizing training shapes. Understanding their memorization could help prevent training data leakage and improve the diversity of generated results. In this paper, we design an evaluation framework to quantify memorization in 3D generative models and study the influence of different data and modeling designs on memorization. We first apply our framework to quantify memorization in existing methods. Next, through controlled experiments with a latent vector-set (Vecset) diffusion model, we find that, on the data side, memorization depends on data modality, and increases with data diversity and finer-grained conditioning; on the modeling side, it peaks at a moderate guidance scale and can be mitigated by longer Vecsets and simple rotation augmentation. Together, our framework and analysis provide an empirical understanding of memorization in 3D generative models and suggest simple yet effective strategies to reduce it without degrading generation quality. Our code is available at https://github.com/zlab-princeton/3d_mem.
Commercial Vehicle Braking Optimization: A Robust SIFT-Trajectory Approach
Dec 21, 2025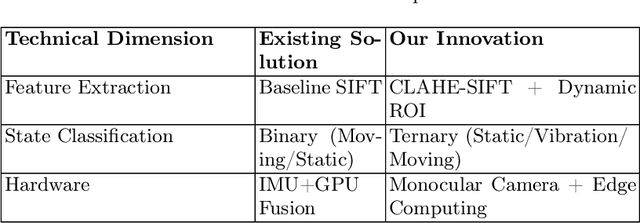
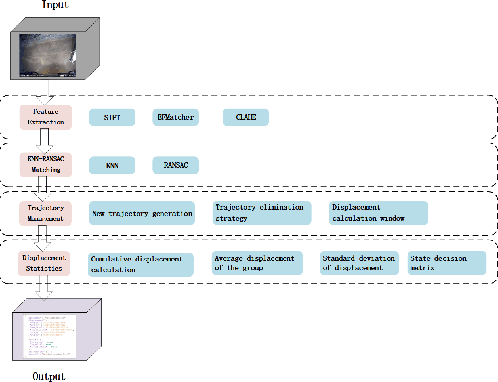

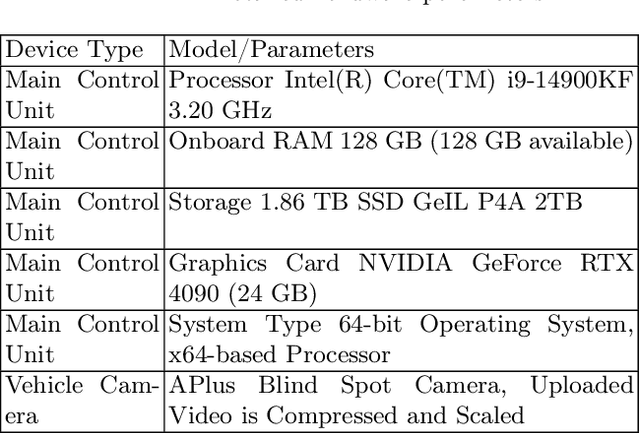
Abstract:A vision-based trajectory analysis solution is proposed to address the "zero-speed braking" issue caused by inaccurate Controller Area Network (CAN) signals in commercial vehicle Automatic Emergency Braking (AEB) systems during low-speed operation. The algorithm utilizes the NVIDIA Jetson AGX Xavier platform to process sequential video frames from a blind spot camera, employing self-adaptive Contrast Limited Adaptive Histogram Equalization (CLAHE)-enhanced Scale-Invariant Feature Transform (SIFT) feature extraction and K-Nearest Neighbors (KNN)-Random Sample Consensus (RANSAC) matching. This allows for precise classification of the vehicle's motion state (static, vibration, moving). Key innovations include 1) multiframe trajectory displacement statistics (5-frame sliding window), 2) a dual-threshold state decision matrix, and 3) OBD-II driven dynamic Region of Interest (ROI) configuration. The system effectively suppresses environmental interference and false detection of dynamic objects, directly addressing the challenge of low-speed false activation in commercial vehicle safety systems. Evaluation in a real-world dataset (32,454 video segments from 1,852 vehicles) demonstrates an F1-score of 99.96% for static detection, 97.78% for moving state recognition, and a processing delay of 14.2 milliseconds (resolution 704x576). The deployment on-site shows an 89% reduction in false braking events, a 100% success rate in emergency braking, and a fault rate below 5%.
SplitFlow: Flow Decomposition for Inversion-Free Text-to-Image Editing
Oct 29, 2025



Abstract:Rectified flow models have become a de facto standard in image generation due to their stable sampling trajectories and high-fidelity outputs. Despite their strong generative capabilities, they face critical limitations in image editing tasks: inaccurate inversion processes for mapping real images back into the latent space, and gradient entanglement issues during editing often result in outputs that do not faithfully reflect the target prompt. Recent efforts have attempted to directly map source and target distributions via ODE-based approaches without inversion; however,these methods still yield suboptimal editing quality. In this work, we propose a flow decomposition-and-aggregation framework built upon an inversion-free formulation to address these limitations. Specifically, we semantically decompose the target prompt into multiple sub-prompts, compute an independent flow for each, and aggregate them to form a unified editing trajectory. While we empirically observe that decomposing the original flow enhances diversity in the target space, generating semantically aligned outputs still requires consistent guidance toward the full target prompt. To this end, we design a projection and soft-aggregation mechanism for flow, inspired by gradient conflict resolution in multi-task learning. This approach adaptively weights the sub-target velocity fields, suppressing semantic redundancy while emphasizing distinct directions, thereby preserving both diversity and consistency in the final edited output. Experimental results demonstrate that our method outperforms existing zero-shot editing approaches in terms of semantic fidelity and attribute disentanglement. The code is available at https://github.com/Harvard-AI-and-Robotics-Lab/SplitFlow.
One More Question is Enough, Expert Question Decomposition (EQD) Model for Domain Quantitative Reasoning
Oct 01, 2025Abstract:Domain-specific quantitative reasoning remains a major challenge for large language models (LLMs), especially in fields requiring expert knowledge and complex question answering (QA). In this work, we propose Expert Question Decomposition (EQD), an approach designed to balance the use of domain knowledge with computational efficiency. EQD is built on a two-step fine-tuning framework and guided by a reward function that measures the effectiveness of generated sub-questions in improving QA outcomes. It requires only a few thousand training examples and a single A100 GPU for fine-tuning, with inference time comparable to zero-shot prompting. Beyond its efficiency, EQD outperforms state-of-the-art domain-tuned models and advanced prompting strategies. We evaluate EQD in the financial domain, characterized by specialized knowledge and complex quantitative reasoning, across four benchmark datasets. Our method consistently improves QA performance by 0.6% to 10.5% across different LLMs. Our analysis reveals an important insight: in domain-specific QA, a single supporting question often provides greater benefit than detailed guidance steps.
All-in-One Slider for Attribute Manipulation in Diffusion Models
Aug 26, 2025Abstract:Text-to-image (T2I) diffusion models have made significant strides in generating high-quality images. However, progressively manipulating certain attributes of generated images to meet the desired user expectations remains challenging, particularly for content with rich details, such as human faces. Some studies have attempted to address this by training slider modules. However, they follow a One-for-One manner, where an independent slider is trained for each attribute, requiring additional training whenever a new attribute is introduced. This not only results in parameter redundancy accumulated by sliders but also restricts the flexibility of practical applications and the scalability of attribute manipulation. To address this issue, we introduce the All-in-One Slider, a lightweight module that decomposes the text embedding space into sparse, semantically meaningful attribute directions. Once trained, it functions as a general-purpose slider, enabling interpretable and fine-grained continuous control over various attributes. Moreover, by recombining the learned directions, the All-in-One Slider supports zero-shot manipulation of unseen attributes (e.g., races and celebrities) and the composition of multiple attributes. Extensive experiments demonstrate that our method enables accurate and scalable attribute manipulation, achieving notable improvements compared to previous methods. Furthermore, our method can be extended to integrate with the inversion framework to perform attribute manipulation on real images, broadening its applicability to various real-world scenarios. The code and trained model will be released at: https://github.com/ywxsuperstar/KSAE-FaceSteer.
CurveFlow: Curvature-Guided Flow Matching for Image Generation
Aug 20, 2025Abstract:Existing rectified flow models are based on linear trajectories between data and noise distributions. This linearity enforces zero curvature, which can inadvertently force the image generation process through low-probability regions of the data manifold. A key question remains underexplored: how does the curvature of these trajectories correlate with the semantic alignment between generated images and their corresponding captions, i.e., instructional compliance? To address this, we introduce CurveFlow, a novel flow matching framework designed to learn smooth, non-linear trajectories by directly incorporating curvature guidance into the flow path. Our method features a robust curvature regularization technique that penalizes abrupt changes in the trajectory's intrinsic dynamics.Extensive experiments on MS COCO 2014 and 2017 demonstrate that CurveFlow achieves state-of-the-art performance in text-to-image generation, significantly outperforming both standard rectified flow variants and other non-linear baselines like Rectified Diffusion. The improvements are especially evident in semantic consistency metrics such as BLEU, METEOR, ROUGE, and CLAIR. This confirms that our curvature-aware modeling substantially enhances the model's ability to faithfully follow complex instructions while simultaneously maintaining high image quality. The code is made publicly available at https://github.com/Harvard-AI-and-Robotics-Lab/CurveFlow.
RingMo-Agent: A Unified Remote Sensing Foundation Model for Multi-Platform and Multi-Modal Reasoning
Jul 28, 2025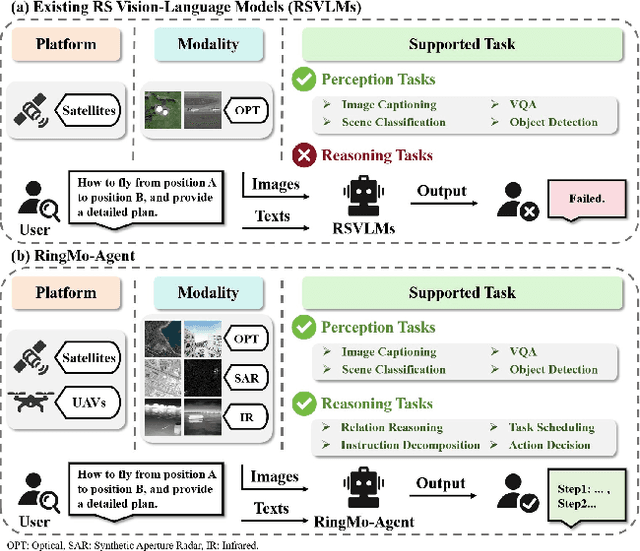
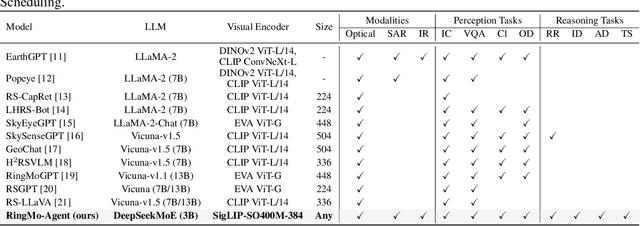

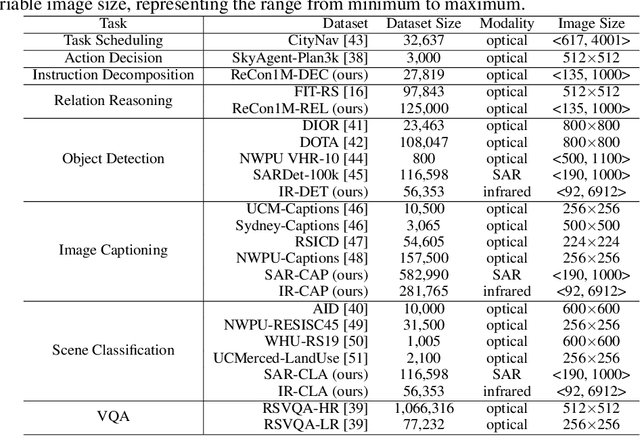
Abstract:Remote sensing (RS) images from multiple modalities and platforms exhibit diverse details due to differences in sensor characteristics and imaging perspectives. Existing vision-language research in RS largely relies on relatively homogeneous data sources. Moreover, they still remain limited to conventional visual perception tasks such as classification or captioning. As a result, these methods fail to serve as a unified and standalone framework capable of effectively handling RS imagery from diverse sources in real-world applications. To address these issues, we propose RingMo-Agent, a model designed to handle multi-modal and multi-platform data that performs perception and reasoning tasks based on user textual instructions. Compared with existing models, RingMo-Agent 1) is supported by a large-scale vision-language dataset named RS-VL3M, comprising over 3 million image-text pairs, spanning optical, SAR, and infrared (IR) modalities collected from both satellite and UAV platforms, covering perception and challenging reasoning tasks; 2) learns modality adaptive representations by incorporating separated embedding layers to construct isolated features for heterogeneous modalities and reduce cross-modal interference; 3) unifies task modeling by introducing task-specific tokens and employing a token-based high-dimensional hidden state decoding mechanism designed for long-horizon spatial tasks. Extensive experiments on various RS vision-language tasks demonstrate that RingMo-Agent not only proves effective in both visual understanding and sophisticated analytical tasks, but also exhibits strong generalizability across different platforms and sensing modalities.
CharaConsist: Fine-Grained Consistent Character Generation
Jul 15, 2025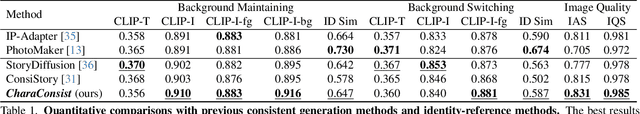

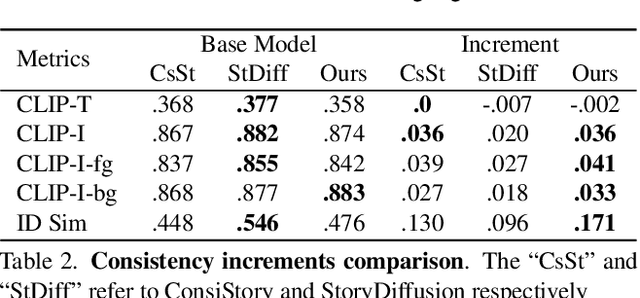

Abstract:In text-to-image generation, producing a series of consistent contents that preserve the same identity is highly valuable for real-world applications. Although a few works have explored training-free methods to enhance the consistency of generated subjects, we observe that they suffer from the following problems. First, they fail to maintain consistent background details, which limits their applicability. Furthermore, when the foreground character undergoes large motion variations, inconsistencies in identity and clothing details become evident. To address these problems, we propose CharaConsist, which employs point-tracking attention and adaptive token merge along with decoupled control of the foreground and background. CharaConsist enables fine-grained consistency for both foreground and background, supporting the generation of one character in continuous shots within a fixed scene or in discrete shots across different scenes. Moreover, CharaConsist is the first consistent generation method tailored for text-to-image DiT model. Its ability to maintain fine-grained consistency, combined with the larger capacity of latest base model, enables it to produce high-quality visual outputs, broadening its applicability to a wider range of real-world scenarios. The source code has been released at https://github.com/Murray-Wang/CharaConsist
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge