M. Ali Nasseri
CLAPS: A CLIP-Unified Auto-Prompt Segmentation for Multi-Modal Retinal Imaging
Sep 10, 2025Abstract:Recent advancements in foundation models, such as the Segment Anything Model (SAM), have significantly impacted medical image segmentation, especially in retinal imaging, where precise segmentation is vital for diagnosis. Despite this progress, current methods face critical challenges: 1) modality ambiguity in textual disease descriptions, 2) a continued reliance on manual prompting for SAM-based workflows, and 3) a lack of a unified framework, with most methods being modality- and task-specific. To overcome these hurdles, we propose CLIP-unified Auto-Prompt Segmentation (\CLAPS), a novel method for unified segmentation across diverse tasks and modalities in retinal imaging. Our approach begins by pre-training a CLIP-based image encoder on a large, multi-modal retinal dataset to handle data scarcity and distribution imbalance. We then leverage GroundingDINO to automatically generate spatial bounding box prompts by detecting local lesions. To unify tasks and resolve ambiguity, we use text prompts enhanced with a unique "modality signature" for each imaging modality. Ultimately, these automated textual and spatial prompts guide SAM to execute precise segmentation, creating a fully automated and unified pipeline. Extensive experiments on 12 diverse datasets across 11 critical segmentation categories show that CLAPS achieves performance on par with specialized expert models while surpassing existing benchmarks across most metrics, demonstrating its broad generalizability as a foundation model.
UOPSL: Unpaired OCT Predilection Sites Learning for Fundus Image Diagnosis Augmentation
Sep 10, 2025Abstract:Significant advancements in AI-driven multimodal medical image diagnosis have led to substantial improvements in ophthalmic disease identification in recent years. However, acquiring paired multimodal ophthalmic images remains prohibitively expensive. While fundus photography is simple and cost-effective, the limited availability of OCT data and inherent modality imbalance hinder further progress. Conventional approaches that rely solely on fundus or textual features often fail to capture fine-grained spatial information, as each imaging modality provides distinct cues about lesion predilection sites. In this study, we propose a novel unpaired multimodal framework \UOPSL that utilizes extensive OCT-derived spatial priors to dynamically identify predilection sites, enhancing fundus image-based disease recognition. Our approach bridges unpaired fundus and OCTs via extended disease text descriptions. Initially, we employ contrastive learning on a large corpus of unpaired OCT and fundus images while simultaneously learning the predilection sites matrix in the OCT latent space. Through extensive optimization, this matrix captures lesion localization patterns within the OCT feature space. During the fine-tuning or inference phase of the downstream classification task based solely on fundus images, where paired OCT data is unavailable, we eliminate OCT input and utilize the predilection sites matrix to assist in fundus image classification learning. Extensive experiments conducted on 9 diverse datasets across 28 critical categories demonstrate that our framework outperforms existing benchmarks.
Pre-Surgical Planner for Robot-Assisted Vitreoretinal Surgery: Integrating Eye Posture, Robot Position and Insertion Point
Feb 25, 2025



Abstract:Several robotic frameworks have been recently developed to assist ophthalmic surgeons in performing complex vitreoretinal procedures such as subretinal injection of advanced therapeutics. These surgical robots show promising capabilities; however, most of them have to limit their working volume to achieve maximum accuracy. Moreover, the visible area seen through the surgical microscope is limited and solely depends on the eye posture. If the eye posture, trocar position, and robot configuration are not correctly arranged, the instrument may not reach the target position, and the preparation will have to be redone. Therefore, this paper proposes the optimization framework of the eye tilting and the robot positioning to reach various target areas for different patients. Our method was validated with an adjustable phantom eye model, and the error of this workflow was 0.13 +/- 1.65 deg (rotational joint around Y axis), -1.40 +/- 1.13 deg (around X axis), and 1.80 +/- 1.51 mm (depth, Z). The potential error sources are also analyzed in the discussion section.
Towards Motion Compensation in Autonomous Robotic Subretinal Injections
Nov 27, 2024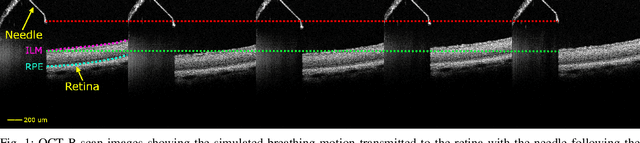

Abstract:Exudative (wet) age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, typically treated with intravitreal injections. Emerging therapies, such as subretinal injections of stem cells, gene therapy, small molecules or RPE cells require precise delivery to avoid damaging delicate retinal structures. Autonomous robotic systems can potentially offer the necessary precision for these procedures. This paper presents a novel approach for motion compensation in robotic subretinal injections, utilizing real-time Optical Coherence Tomography (OCT). The proposed method leverages B$^{5}$-scans, a rapid acquisition of small-volume OCT data, for dynamic tracking of retinal motion along the Z-axis, compensating for physiological movements such as breathing and heartbeat. Validation experiments on \textit{ex vivo} porcine eyes revealed challenges in maintaining a consistent tool-to-retina distance, with deviations of up to 200 $\mu m$ for 100 $\mu m$ amplitude motions and over 80 $\mu m$ for 25 $\mu m$ amplitude motions over one minute. Subretinal injections faced additional difficulties, with horizontal shifts causing the needle to move off-target and inject into the vitreous. These results highlight the need for improved motion prediction and horizontal stability to enhance the accuracy and safety of robotic subretinal procedures.
Real-time Deformation-aware Control for Autonomous Robotic Subretinal Injection under iOCT Guidance
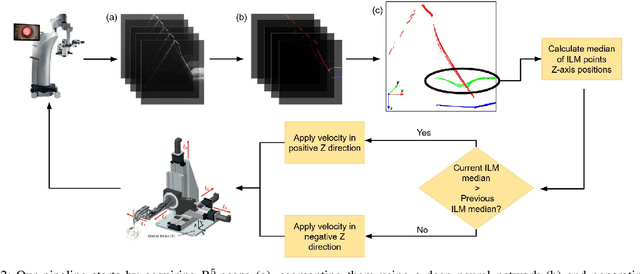
Nov 10, 2024



Abstract:Robotic platforms provide repeatable and precise tool positioning that significantly enhances retinal microsurgery. Integration of such systems with intraoperative optical coherence tomography (iOCT) enables image-guided robotic interventions, allowing to autonomously perform advanced treatment possibilities, such as injecting therapeutic agents into the subretinal space. Yet, tissue deformations due to tool-tissue interactions are a major challenge in autonomous iOCT-guided robotic subretinal injection, impacting correct needle positioning and, thus, the outcome of the procedure. This paper presents a novel method for autonomous subretinal injection under iOCT guidance that considers tissue deformations during the insertion procedure. This is achieved through real-time segmentation and 3D reconstruction of the surgical scene from densely sampled iOCT B-scans, which we refer to as B5-scans, to monitor the positioning of the instrument regarding a virtual target layer defined at a relative position between the ILM and RPE. Our experiments on ex-vivo porcine eyes demonstrate dynamic adjustment of the insertion depth and overall improved accuracy in needle positioning compared to previous autonomous insertion approaches. Compared to a 35% success rate in subretinal bleb generation with previous approaches, our proposed method reliably and robustly created subretinal blebs in all our experiments.
Extrapolating Prospective Glaucoma Fundus Images through Diffusion Model in Irregular Longitudinal Sequences
Oct 28, 2024



Abstract:The utilization of longitudinal datasets for glaucoma progression prediction offers a compelling approach to support early therapeutic interventions. Predominant methodologies in this domain have primarily focused on the direct prediction of glaucoma stage labels from longitudinal datasets. However, such methods may not adequately encapsulate the nuanced developmental trajectory of the disease. To enhance the diagnostic acumen of medical practitioners, we propose a novel diffusion-based model to predict prospective images by extrapolating from existing longitudinal fundus images of patients. The methodology delineated in this study distinctively leverages sequences of images as inputs. Subsequently, a time-aligned mask is employed to select a specific year for image generation. During the training phase, the time-aligned mask resolves the issue of irregular temporal intervals in longitudinal image sequence sampling. Additionally, we utilize a strategy of randomly masking a frame in the sequence to establish the ground truth. This methodology aids the network in continuously acquiring knowledge regarding the internal relationships among the sequences throughout the learning phase. Moreover, the introduction of textual labels is instrumental in categorizing images generated within the sequence. The empirical findings from the conducted experiments indicate that our proposed model not only effectively generates longitudinal data but also significantly improves the precision of downstream classification tasks.
KaLDeX: Kalman Filter based Linear Deformable Cross Attention for Retina Vessel Segmentation
Oct 28, 2024Abstract:Background and Objective: In the realm of ophthalmic imaging, accurate vascular segmentation is paramount for diagnosing and managing various eye diseases. Contemporary deep learning-based vascular segmentation models rival human accuracy but still face substantial challenges in accurately segmenting minuscule blood vessels in neural network applications. Due to the necessity of multiple downsampling operations in the CNN models, fine details from high-resolution images are inevitably lost. The objective of this study is to design a structure to capture the delicate and small blood vessels. Methods: To address these issues, we propose a novel network (KaLDeX) for vascular segmentation leveraging a Kalman filter based linear deformable cross attention (LDCA) module, integrated within a UNet++ framework. Our approach is based on two key components: Kalman filter (KF) based linear deformable convolution (LD) and cross-attention (CA) modules. The LD module is designed to adaptively adjust the focus on thin vessels that might be overlooked in standard convolution. The CA module improves the global understanding of vascular structures by aggregating the detailed features from the LD module with the high level features from the UNet++ architecture. Finally, we adopt a topological loss function based on persistent homology to constrain the topological continuity of the segmentation. Results: The proposed method is evaluated on retinal fundus image datasets (DRIVE, CHASE_BD1, and STARE) as well as the 3mm and 6mm of the OCTA-500 dataset, achieving an average accuracy (ACC) of 97.25%, 97.77%, 97.85%, 98.89%, and 98.21%, respectively. Conclusions: Empirical evidence shows that our method outperforms the current best models on different vessel segmentation datasets. Our source code is available at: https://github.com/AIEyeSystem/KalDeX.
AI-Based Fully Automatic Analysis of Retinal Vascular Morphology in Pediatric High Myopia
Sep 30, 2024Abstract:Purpose: To investigate the changes in retinal vascular structures associated various stages of myopia by designing automated software based on an artif intelligencemodel. Methods: The study involved 1324 pediatric participants from the National Childr Medical Center in China, and 2366 high-quality retinal images and correspon refractive parameters were obtained and analyzed. Spherical equivalent refrac(SER) degree was calculated. We proposed a data analysis model based c combination of the Convolutional Neural Networks (CNN) model and the atter module to classify images, segment vascular structures, and measure vasc parameters, such as main angle (MA), branching angle (BA), bifurcation edge al(BEA) and bifurcation edge coefficient (BEC). One-way ANOVA compared param measurements betweenthenormalfundus,lowmyopia,moderate myopia,and high myopia group. Results: There were 279 (12.38%) images in normal group and 384 (16.23%) images in the high myopia group. Compared normal fundus, the MA of fundus vessels in different myopic refractive groups significantly reduced (P = 0.006, P = 0.004, P = 0.019, respectively), and performance of the venous system was particularly obvious (P<0.001). At the sa time, the BEC decreased disproportionately (P<0.001). Further analysis of fundus vascular parameters at different degrees of myopia showed that there were also significant differences in BA and branching coefficient (BC). The arterial BA value of the fundus vessel in the high myopia group was lower than that of other groups (P : 0.032, 95% confidence interval [Ci], 0.22-4.86), while the venous BA values increased(P = 0.026). The BEC values of high myopia were higher than those of low and moderate myopia groups. When the loss function of our data classification model converged to 0.09,the model accuracy reached 94.19%
EyeLS: Shadow-Guided Instrument Landing System for Intraocular Target Approaching in Robotic Eye Surgery
Nov 15, 2023Abstract:Robotic ophthalmic surgery is an emerging technology to facilitate high-precision interventions such as retina penetration in subretinal injection and removal of floating tissues in retinal detachment depending on the input imaging modalities such as microscopy and intraoperative OCT (iOCT). Although iOCT is explored to locate the needle tip within its range-limited ROI, it is still difficult to coordinate iOCT's motion with the needle, especially at the initial target-approaching stage. Meanwhile, due to 2D perspective projection and thus the loss of depth information, current image-based methods cannot effectively estimate the needle tip's trajectory towards both retinal and floating targets. To address this limitation, we propose to use the shadow positions of the target and the instrument tip to estimate their relative depth position and accordingly optimize the instrument tip's insertion trajectory until the tip approaches targets within iOCT's scanning area. Our method succeeds target approaching on a retina model, and achieves an average depth error of 0.0127 mm and 0.3473 mm for floating and retinal targets respectively in the surgical simulator without damaging the retina.
Robotic Navigation Autonomy for Subretinal Injection via Intelligent Real-Time Virtual iOCT Volume Slicing
Jan 17, 2023Abstract:In the last decade, various robotic platforms have been introduced that could support delicate retinal surgeries. Concurrently, to provide semantic understanding of the surgical area, recent advances have enabled microscope-integrated intraoperative Optical Coherent Tomography (iOCT) with high-resolution 3D imaging at near video rate. The combination of robotics and semantic understanding enables task autonomy in robotic retinal surgery, such as for subretinal injection. This procedure requires precise needle insertion for best treatment outcomes. However, merging robotic systems with iOCT introduces new challenges. These include, but are not limited to high demands on data processing rates and dynamic registration of these systems during the procedure. In this work, we propose a framework for autonomous robotic navigation for subretinal injection, based on intelligent real-time processing of iOCT volumes. Our method consists of an instrument pose estimation method, an online registration between the robotic and the iOCT system, and trajectory planning tailored for navigation to an injection target. We also introduce intelligent virtual B-scans, a volume slicing approach for rapid instrument pose estimation, which is enabled by Convolutional Neural Networks (CNNs). Our experiments on ex-vivo porcine eyes demonstrate the precision and repeatability of the method. Finally, we discuss identified challenges in this work and suggest potential solutions to further the development of such systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge