Iulian Iordachita
An Extended Generalized Prandtl-Ishlinskii Hysteresis Model for I2RIS Robot
Apr 16, 2025Abstract:Retinal surgery requires extreme precision due to constrained anatomical spaces in the human retina. To assist surgeons achieve this level of accuracy, the Improved Integrated Robotic Intraocular Snake (I2RIS) with dexterous capability has been developed. However, such flexible tendon-driven robots often suffer from hysteresis problems, which significantly challenges precise control and positioning. In particular, we observed multi-stage hysteresis phenomena in the small-scale I2RIS. In this paper, we propose an Extended Generalized Prandtl-Ishlinskii (EGPI) model to increase the fitting accuracy of the hysteresis. The model incorporates a novel switching mechanism that enables it to describe multi-stage hysteresis in the regions of monotonic input. Experimental validation on I2RIS data demonstrate that the EGPI model outperforms the conventional Generalized Prandtl-Ishlinskii (GPI) model in terms of RMSE, NRMSE, and MAE across multiple motor input directions. The EGPI model in our study highlights the potential in modeling multi-stage hysteresis in minimally invasive flexible robots.
Deep Learning-Enhanced Robotic Subretinal Injection with Real-Time Retinal Motion Compensation
Apr 04, 2025Abstract:Subretinal injection is a critical procedure for delivering therapeutic agents to treat retinal diseases such as age-related macular degeneration (AMD). However, retinal motion caused by physiological factors such as respiration and heartbeat significantly impacts precise needle positioning, increasing the risk of retinal pigment epithelium (RPE) damage. This paper presents a fully autonomous robotic subretinal injection system that integrates intraoperative optical coherence tomography (iOCT) imaging and deep learning-based motion prediction to synchronize needle motion with retinal displacement. A Long Short-Term Memory (LSTM) neural network is used to predict internal limiting membrane (ILM) motion, outperforming a Fast Fourier Transform (FFT)-based baseline model. Additionally, a real-time registration framework aligns the needle tip position with the robot's coordinate frame. Then, a dynamic proportional speed control strategy ensures smooth and adaptive needle insertion. Experimental validation in both simulation and ex vivo open-sky porcine eyes demonstrates precise motion synchronization and successful subretinal injections. The experiment achieves a mean tracking error below 16.4 {\mu}m in pre-insertion phases. These results show the potential of AI-driven robotic assistance to improve the safety and accuracy of retinal microsurgery.
Model Predictive Path Integral Control of I2RIS Robot Using RBF Identifier and Extended Kalman Filter
Mar 18, 2025Abstract:Modeling and controlling cable-driven snake robots is a challenging problem due to nonlinear mechanical properties such as hysteresis, variable stiffness, and unknown friction between the actuation cables and the robot body. This challenge is more significant for snake robots in ophthalmic surgery applications, such as the Improved Integrated Robotic Intraocular Snake (I$^2$RIS), given its small size and lack of embedded sensory feedback. Data-driven models take advantage of global function approximations, reducing complicated analytical models' challenge and computational costs. However, their performance might deteriorate in case of new data unseen in the training phase. Therefore, adding an adaptation mechanism might improve these models' performance during snake robots' interactions with unknown environments. In this work, we applied a model predictive path integral (MPPI) controller on a data-driven model of the I$^2$RIS based on the Gaussian mixture model (GMM) and Gaussian mixture regression (GMR). To analyze the performance of the MPPI in unseen robot-tissue interaction situations, unknown external disturbances and environmental loads are simulated and added to the GMM-GMR model. These uncertainties of the robot model are then identified online using a radial basis function (RBF) whose weights are updated using an extended Kalman filter (EKF). Simulation results demonstrated the robustness of the optimal control solutions of the MPPI algorithm and its computational superiority over a conventional model predictive control (MPC) algorithm.
A Feasible Workflow for Retinal Vein Cannulation in Ex Vivo Porcine Eyes with Robotic Assistance
Dec 16, 2024



Abstract:A potential Retinal Vein Occlusion (RVO) treatment involves Retinal Vein Cannulation (RVC), which requires the surgeon to insert a microneedle into the affected retinal vein and administer a clot-dissolving drug. This procedure presents significant challenges due to human physiological limitations, such as hand tremors, prolonged tool-holding periods, and constraints in depth perception using a microscope. This study proposes a robot-assisted workflow for RVC to overcome these limitations. The test robot is operated through a keyboard. An intraoperative Optical Coherence Tomography (iOCT) system is used to verify successful venous puncture before infusion. The workflow is validated using 12 ex vivo porcine eyes. These early results demonstrate a successful rate of 10 out of 12 cannulations (83.33%), affirming the feasibility of the proposed workflow.
Towards Motion Compensation in Autonomous Robotic Subretinal Injections
Nov 27, 2024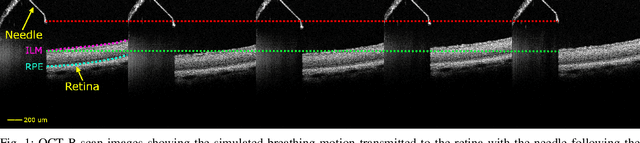
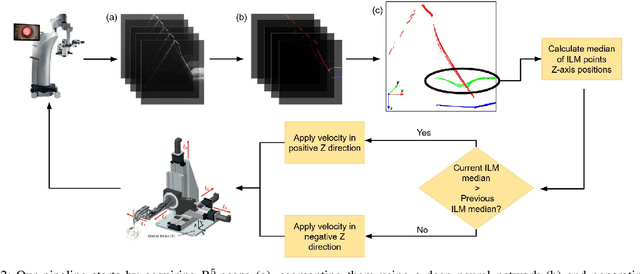

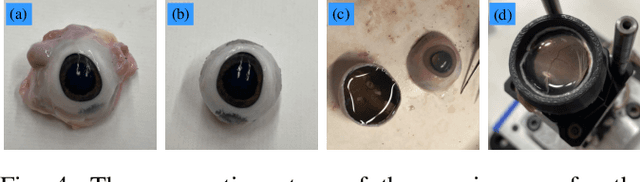
Abstract:Exudative (wet) age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, typically treated with intravitreal injections. Emerging therapies, such as subretinal injections of stem cells, gene therapy, small molecules or RPE cells require precise delivery to avoid damaging delicate retinal structures. Autonomous robotic systems can potentially offer the necessary precision for these procedures. This paper presents a novel approach for motion compensation in robotic subretinal injections, utilizing real-time Optical Coherence Tomography (OCT). The proposed method leverages B$^{5}$-scans, a rapid acquisition of small-volume OCT data, for dynamic tracking of retinal motion along the Z-axis, compensating for physiological movements such as breathing and heartbeat. Validation experiments on \textit{ex vivo} porcine eyes revealed challenges in maintaining a consistent tool-to-retina distance, with deviations of up to 200 $\mu m$ for 100 $\mu m$ amplitude motions and over 80 $\mu m$ for 25 $\mu m$ amplitude motions over one minute. Subretinal injections faced additional difficulties, with horizontal shifts causing the needle to move off-target and inject into the vitreous. These results highlight the need for improved motion prediction and horizontal stability to enhance the accuracy and safety of robotic subretinal procedures.
Minimally Invasive Flexible Needle Manipulation Based on Finite Element Simulation and Cross Entropy Method
Nov 12, 2024



Abstract:We present a novel approach for minimally invasive flexible needle manipulations by pairing a real-time finite element simulator with the cross-entropy method. Additionally, we demonstrate how a kinematic-driven bang-bang controller can complement the control framework for better tracking performance. We show how electromagnetic (EM) tracking can be readily incorporated into the framework to provide controller feedback. Tissue phantom experiment with EM tracking shows the average targeting error is $0.16 \pm 0.29mm$.
Real-time Deformation-aware Control for Autonomous Robotic Subretinal Injection under iOCT Guidance
Nov 10, 2024



Abstract:Robotic platforms provide repeatable and precise tool positioning that significantly enhances retinal microsurgery. Integration of such systems with intraoperative optical coherence tomography (iOCT) enables image-guided robotic interventions, allowing to autonomously perform advanced treatment possibilities, such as injecting therapeutic agents into the subretinal space. Yet, tissue deformations due to tool-tissue interactions are a major challenge in autonomous iOCT-guided robotic subretinal injection, impacting correct needle positioning and, thus, the outcome of the procedure. This paper presents a novel method for autonomous subretinal injection under iOCT guidance that considers tissue deformations during the insertion procedure. This is achieved through real-time segmentation and 3D reconstruction of the surgical scene from densely sampled iOCT B-scans, which we refer to as B5-scans, to monitor the positioning of the instrument regarding a virtual target layer defined at a relative position between the ILM and RPE. Our experiments on ex-vivo porcine eyes demonstrate dynamic adjustment of the insertion depth and overall improved accuracy in needle positioning compared to previous autonomous insertion approaches. Compared to a 35% success rate in subretinal bleb generation with previous approaches, our proposed method reliably and robustly created subretinal blebs in all our experiments.
Bimanual Manipulation of Steady Hand Eye Robots with Adaptive Sclera Force Control: Cooperative vs. Teleoperation Strategies
Feb 28, 2024



Abstract:Performing intricate eye microsurgery, such as retinal vein cannulation (RVC), as a potential treatment for retinal vein occlusion (RVO), without the assistance of a surgical robotic system is very challenging to do safely. The main limitation has to do with the physiological hand tremor of surgeons. Robot-assisted eye surgery technology may resolve the problems of hand tremors and fatigue and improve the safety and precision of RVC. The Steady-Hand Eye Robot (SHER) is an admittance-based robotic system that can filter out hand tremors and enables ophthalmologists to manipulate a surgical instrument inside the eye cooperatively. However, the admittance-based cooperative control mode does not address crucial safety considerations, such as minimizing contact force between the surgical instrument and the sclera surface to prevent tissue damage. An adaptive sclera force control algorithm was proposed to address this limitation using an FBG-based force-sensing tool to measure and minimize the tool-sclera interaction force. Additionally, features like haptic feedback or hand motion scaling, which can improve the safety and precision of surgery, require a teleoperation control framework. We implemented a bimanual adaptive teleoperation (BMAT) control mode using SHER 2.0 and SHER 2.1 and compared its performance with a bimanual adaptive cooperative (BMAC) mode. Both BMAT and BMAC modes were tested in sitting and standing postures during a vessel-following experiment under a surgical microscope. It is shown, for the first time to the best of our knowledge in robot-assisted retinal surgery, that integrating the adaptive sclera force control algorithm with the bimanual teleoperation framework enables surgeons to safely perform bimanual telemanipulation of the eye without over-stretching it, even in the absence of registration between the two robots.
Shape Manipulation of Bevel-Tip Needles for Prostate Biopsy Procedures: A Comparison of Two Resolved-Rate Controllers
Feb 05, 2024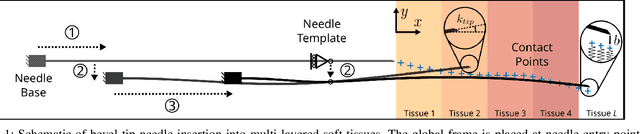
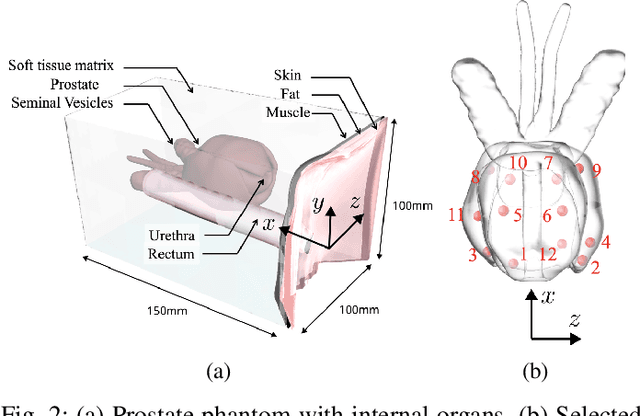
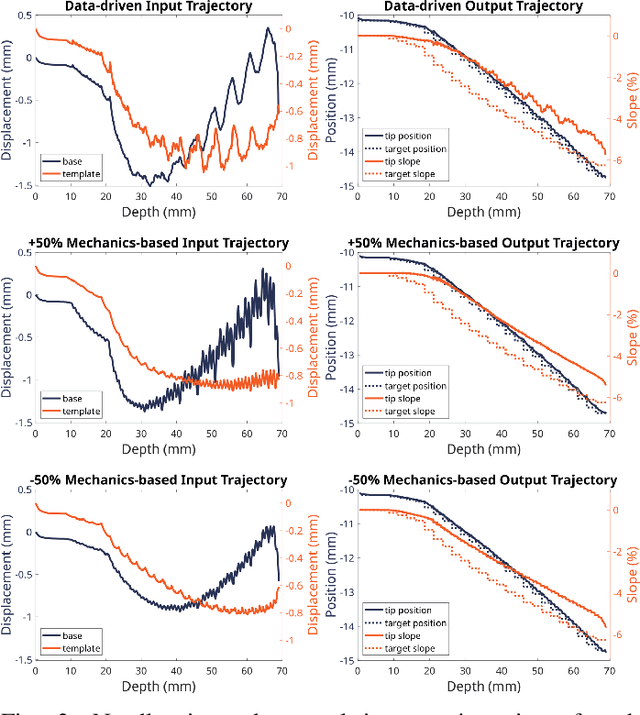

Abstract:Prostate cancer diagnosis continues to encounter challenges, often due to imprecise needle placement in standard biopsies. Several control strategies have been developed to compensate for needle tip prediction inaccuracies, however none were compared against each other, and it is unclear whether any of them can be safely and universally applied in clinical settings. This paper compares the performance of two resolved-rate controllers, derived from a mechanics-based and a data-driven approach, for bevel-tip needle control using needle shape manipulation through a template. We demonstrate for a simulated 12-core biopsy procedure under model parameter uncertainty that the mechanics-based controller can better reach desired targets when only the final goal configuration is presented even with uncertainty on model parameters estimation, and that providing a feasible needle path is crucial in ensuring safe surgical outcomes when either controller is used for needle shape manipulation.
Cooperative vs. Teleoperation Control of the Steady Hand Eye Robot with Adaptive Sclera Force Control: A Comparative Study
Dec 04, 2023Abstract:A surgeon's physiological hand tremor can significantly impact the outcome of delicate and precise retinal surgery, such as retinal vein cannulation (RVC) and epiretinal membrane peeling. Robot-assisted eye surgery technology provides ophthalmologists with advanced capabilities such as hand tremor cancellation, hand motion scaling, and safety constraints that enable them to perform these otherwise challenging and high-risk surgeries with high precision and safety. Steady-Hand Eye Robot (SHER) with cooperative control mode can filter out surgeon's hand tremor, yet another important safety feature, that is, minimizing the contact force between the surgical instrument and sclera surface for avoiding tissue damage cannot be met in this control mode. Also, other capabilities, such as hand motion scaling and haptic feedback, require a teleoperation control framework. In this work, for the first time, we implemented a teleoperation control mode incorporated with an adaptive sclera force control algorithm using a PHANTOM Omni haptic device and a force-sensing surgical instrument equipped with Fiber Bragg Grating (FBG) sensors attached to the SHER 2.1 end-effector. This adaptive sclera force control algorithm allows the robot to dynamically minimize the tool-sclera contact force. Moreover, for the first time, we compared the performance of the proposed adaptive teleoperation mode with the cooperative mode by conducting a vessel-following experiment inside an eye phantom under a microscope.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge