Peiyao Zhang
A Deep Learning-Driven Autonomous System for Retinal Vein Cannulation: Validation Using a Chicken Embryo Model
Jul 29, 2025Abstract:Retinal vein cannulation (RVC) is a minimally invasive microsurgical procedure for treating retinal vein occlusion (RVO), a leading cause of vision impairment. However, the small size and fragility of retinal veins, coupled with the need for high-precision, tremor-free needle manipulation, create significant technical challenges. These limitations highlight the need for robotic assistance to improve accuracy and stability. This study presents an automated robotic system with a top-down microscope and B-scan optical coherence tomography (OCT) imaging for precise depth sensing. Deep learning-based models enable real-time needle navigation, contact detection, and vein puncture recognition, using a chicken embryo model as a surrogate for human retinal veins. The system autonomously detects needle position and puncture events with 85% accuracy. The experiments demonstrate notable reductions in navigation and puncture times compared to manual methods. Our results demonstrate the potential of integrating advanced imaging and deep learning to automate microsurgical tasks, providing a pathway for safer and more reliable RVC procedures with enhanced precision and reproducibility.
Deep Learning-Enhanced Robotic Subretinal Injection with Real-Time Retinal Motion Compensation
Apr 04, 2025Abstract:Subretinal injection is a critical procedure for delivering therapeutic agents to treat retinal diseases such as age-related macular degeneration (AMD). However, retinal motion caused by physiological factors such as respiration and heartbeat significantly impacts precise needle positioning, increasing the risk of retinal pigment epithelium (RPE) damage. This paper presents a fully autonomous robotic subretinal injection system that integrates intraoperative optical coherence tomography (iOCT) imaging and deep learning-based motion prediction to synchronize needle motion with retinal displacement. A Long Short-Term Memory (LSTM) neural network is used to predict internal limiting membrane (ILM) motion, outperforming a Fast Fourier Transform (FFT)-based baseline model. Additionally, a real-time registration framework aligns the needle tip position with the robot's coordinate frame. Then, a dynamic proportional speed control strategy ensures smooth and adaptive needle insertion. Experimental validation in both simulation and ex vivo open-sky porcine eyes demonstrates precise motion synchronization and successful subretinal injections. The experiment achieves a mean tracking error below 16.4 {\mu}m in pre-insertion phases. These results show the potential of AI-driven robotic assistance to improve the safety and accuracy of retinal microsurgery.
A Feasible Workflow for Retinal Vein Cannulation in Ex Vivo Porcine Eyes with Robotic Assistance
Dec 16, 2024



Abstract:A potential Retinal Vein Occlusion (RVO) treatment involves Retinal Vein Cannulation (RVC), which requires the surgeon to insert a microneedle into the affected retinal vein and administer a clot-dissolving drug. This procedure presents significant challenges due to human physiological limitations, such as hand tremors, prolonged tool-holding periods, and constraints in depth perception using a microscope. This study proposes a robot-assisted workflow for RVC to overcome these limitations. The test robot is operated through a keyboard. An intraoperative Optical Coherence Tomography (iOCT) system is used to verify successful venous puncture before infusion. The workflow is validated using 12 ex vivo porcine eyes. These early results demonstrate a successful rate of 10 out of 12 cannulations (83.33%), affirming the feasibility of the proposed workflow.
Towards Motion Compensation in Autonomous Robotic Subretinal Injections
Nov 27, 2024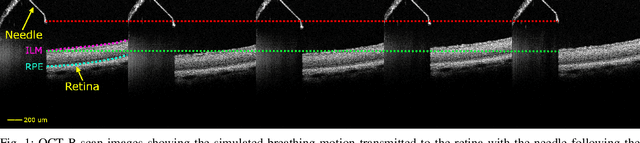
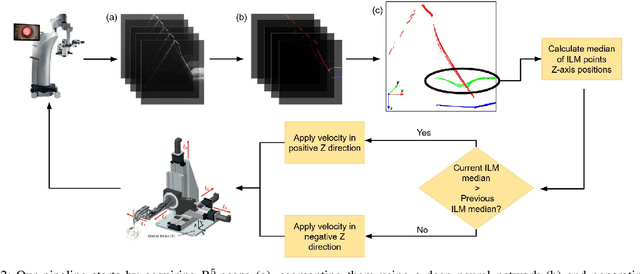

Abstract:Exudative (wet) age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, typically treated with intravitreal injections. Emerging therapies, such as subretinal injections of stem cells, gene therapy, small molecules or RPE cells require precise delivery to avoid damaging delicate retinal structures. Autonomous robotic systems can potentially offer the necessary precision for these procedures. This paper presents a novel approach for motion compensation in robotic subretinal injections, utilizing real-time Optical Coherence Tomography (OCT). The proposed method leverages B$^{5}$-scans, a rapid acquisition of small-volume OCT data, for dynamic tracking of retinal motion along the Z-axis, compensating for physiological movements such as breathing and heartbeat. Validation experiments on \textit{ex vivo} porcine eyes revealed challenges in maintaining a consistent tool-to-retina distance, with deviations of up to 200 $\mu m$ for 100 $\mu m$ amplitude motions and over 80 $\mu m$ for 25 $\mu m$ amplitude motions over one minute. Subretinal injections faced additional difficulties, with horizontal shifts causing the needle to move off-target and inject into the vitreous. These results highlight the need for improved motion prediction and horizontal stability to enhance the accuracy and safety of robotic subretinal procedures.
Real-time Deformation-aware Control for Autonomous Robotic Subretinal Injection under iOCT Guidance
Nov 10, 2024



Abstract:Robotic platforms provide repeatable and precise tool positioning that significantly enhances retinal microsurgery. Integration of such systems with intraoperative optical coherence tomography (iOCT) enables image-guided robotic interventions, allowing to autonomously perform advanced treatment possibilities, such as injecting therapeutic agents into the subretinal space. Yet, tissue deformations due to tool-tissue interactions are a major challenge in autonomous iOCT-guided robotic subretinal injection, impacting correct needle positioning and, thus, the outcome of the procedure. This paper presents a novel method for autonomous subretinal injection under iOCT guidance that considers tissue deformations during the insertion procedure. This is achieved through real-time segmentation and 3D reconstruction of the surgical scene from densely sampled iOCT B-scans, which we refer to as B5-scans, to monitor the positioning of the instrument regarding a virtual target layer defined at a relative position between the ILM and RPE. Our experiments on ex-vivo porcine eyes demonstrate dynamic adjustment of the insertion depth and overall improved accuracy in needle positioning compared to previous autonomous insertion approaches. Compared to a 35% success rate in subretinal bleb generation with previous approaches, our proposed method reliably and robustly created subretinal blebs in all our experiments.
Micromanipulation in Surgery: Autonomous Needle Insertion Inside the Eye for Targeted Drug Delivery
Jun 30, 2023Abstract:We consider a micromanipulation problem in eye surgery, specifically retinal vein cannulation (RVC). RVC involves inserting a microneedle into a retinal vein for the purpose of targeted drug delivery. The procedure requires accurately guiding a needle to a target vein and inserting it while avoiding damage to the surrounding tissues. RVC can be considered similar to the reach or push task studied in robotics manipulation, but with additional constraints related to precision and safety while interacting with soft tissues. Prior works have mainly focused developing robotic hardware and sensors to enhance the surgeons' accuracy, leaving the automation of RVC largely unexplored. In this paper, we present the first autonomous strategy for RVC while relying on a minimal setup: a robotic arm, a needle, and monocular images. Our system exclusively relies on monocular vision to achieve precise navigation, gentle placement on the target vein, and safe insertion without causing tissue damage. Throughout the procedure, we employ machine learning for perception and to identify key surgical events such as needle-vein contact and vein punctures. Detecting these events guides our task and motion planning framework, which generates safe trajectories using model predictive control to complete the procedure. We validate our system through 24 successful autonomous trials on 4 cadaveric pig eyes. We show that our system can navigate to target veins within 22 micrometers of XY accuracy and under 35 seconds, and consistently puncture the target vein without causing tissue damage. Preliminary comparison to a human demonstrates the superior accuracy and reliability of our system.
Deep Learning Guided Autonomous Retinal Surgery using a Robotic Arm, Microscopy, and iOCT Imaging
Jun 16, 2023



Abstract:Recent technological advancements in retinal surgery has led to the modern operating room consisting of a surgical robot, microscope, and intraoperative optical coherence tomography (iOCT). The integration of these tools raises the fundamental question of how to effectively combine them to enable surgical autonomy. In this work, we address this question by developing a unified framework that enables real-time autonomous surgical workflows utilizing the aforementioned devices. To achieve this, we make the following contributions: (1) we develop a novel imaging system that integrates microscopy and iOCT in real-time, accomplished by dynamically tracking the surgical instrument via a small iOCT scanning region (e.g. B-scan), which was not previously possible; (2) implementing various convolutional neural networks (CNN) that automatically segment and detect task-relevant information for surgical autonomy; (3) enabling surgeons to intuitively select goal waypoints within both the microscope and iOCT views through simple mouse-click interactions; (4) integrating model predictive control (MPC) for real-time trajectory generation that respects kinematic constraints to ensure patient safety. We show the utility of our system by tackling subretinal injection (SI), a challenging procedure that involves inserting a microneedle below the retinal tissue for targeted drug delivery, a task surgeons find challenging due to requiring tens-of-micrometers of accuracy and precise depth perception. We validate our system by conducting 30 successful SI trials on pig eyes, achieving needle insertion accuracy of $26 \pm 12 \mu m$ to various subretinal goals and duration of $55 \pm 10.8$ seconds. Preliminary comparisons to a human operator performing SI in robot-assisted mode highlight the enhanced safety of our system.
Deep Learning Guided Autonomous Surgery: Guiding Small Needles into Sub-Millimeter Scale Blood Vessels
Jun 16, 2023



Abstract:We propose a general strategy for autonomous guidance and insertion of a needle into a retinal blood vessel. The main challenges underpinning this task are the accurate placement of the needle-tip on the target vein and a careful needle insertion maneuver to avoid double-puncturing the vein, while dealing with challenging kinematic constraints and depth-estimation uncertainty. Following how surgeons perform this task purely based on visual feedback, we develop a system which relies solely on \emph{monocular} visual cues by combining data-driven kinematic and contact estimation, visual-servoing, and model-based optimal control. By relying on both known kinematic models, as well as deep-learning based perception modules, the system can localize the surgical needle tip and detect needle-tissue interactions and venipuncture events. The outputs from these perception modules are then combined with a motion planning framework that uses visual-servoing and optimal control to cannulate the target vein, while respecting kinematic constraints that consider the safety of the procedure. We demonstrate that we can reliably and consistently perform needle insertion in the domain of retinal surgery, specifically in performing retinal vein cannulation. Using cadaveric pig eyes, we demonstrate that our system can navigate to target veins within 22$\mu m$ XY accuracy and perform the entire procedure in less than 35 seconds on average, and all 24 trials performed on 4 pig eyes were successful. Preliminary comparison study against a human operator show that our system is consistently more accurate and safer, especially during safety-critical needle-tissue interactions. To the best of the authors' knowledge, this work accomplishes a first demonstration of autonomous retinal vein cannulation at a clinically-relevant setting using animal tissues.
Autonomous Needle Navigation in Retinal Microsurgery: Evaluation in ex vivo Porcine Eyes
Jan 27, 2023Abstract:Important challenges in retinal microsurgery include prolonged operating time, inadequate force feedback, and poor depth perception due to a constrained top-down view of the surgery. The introduction of robot-assisted technology could potentially deal with such challenges and improve the surgeon's performance. Motivated by such challenges, this work develops a strategy for autonomous needle navigation in retinal microsurgery aiming to achieve precise manipulation, reduced end-to-end surgery time, and enhanced safety. This is accomplished through real-time geometry estimation and chance-constrained Model Predictive Control (MPC) resulting in high positional accuracy while keeping scleral forces within a safe level. The robotic system is validated using both open-sky and intact (with lens and partial vitreous removal) ex vivo porcine eyes. The experimental results demonstrate that the generation of safe control trajectories is robust to small motions associated with head drift. The mean navigation time and scleral force for MPC navigation experiments are 7.208 s and 11.97 mN, which can be considered efficient and well within acceptable safe limits. The resulting mean errors along lateral directions of the retina are below 0.06 mm, which is below the typical hand tremor amplitude in retinal microsurgery.
Robotic Navigation Autonomy for Subretinal Injection via Intelligent Real-Time Virtual iOCT Volume Slicing
Jan 17, 2023Abstract:In the last decade, various robotic platforms have been introduced that could support delicate retinal surgeries. Concurrently, to provide semantic understanding of the surgical area, recent advances have enabled microscope-integrated intraoperative Optical Coherent Tomography (iOCT) with high-resolution 3D imaging at near video rate. The combination of robotics and semantic understanding enables task autonomy in robotic retinal surgery, such as for subretinal injection. This procedure requires precise needle insertion for best treatment outcomes. However, merging robotic systems with iOCT introduces new challenges. These include, but are not limited to high demands on data processing rates and dynamic registration of these systems during the procedure. In this work, we propose a framework for autonomous robotic navigation for subretinal injection, based on intelligent real-time processing of iOCT volumes. Our method consists of an instrument pose estimation method, an online registration between the robotic and the iOCT system, and trajectory planning tailored for navigation to an injection target. We also introduce intelligent virtual B-scans, a volume slicing approach for rapid instrument pose estimation, which is enabled by Convolutional Neural Networks (CNNs). Our experiments on ex-vivo porcine eyes demonstrate the precision and repeatability of the method. Finally, we discuss identified challenges in this work and suggest potential solutions to further the development of such systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge