Li-wei H. Lehman
A Knowledge Distillation Approach for Sepsis Outcome Prediction from Multivariate Clinical Time Series
Nov 16, 2023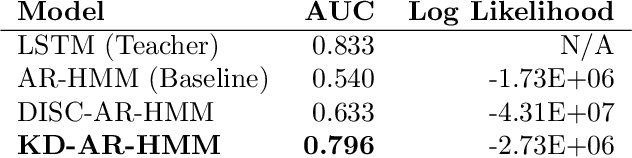
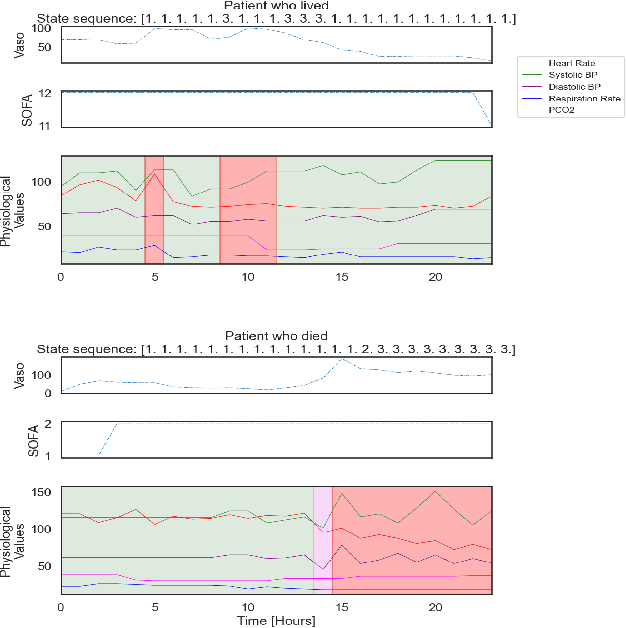
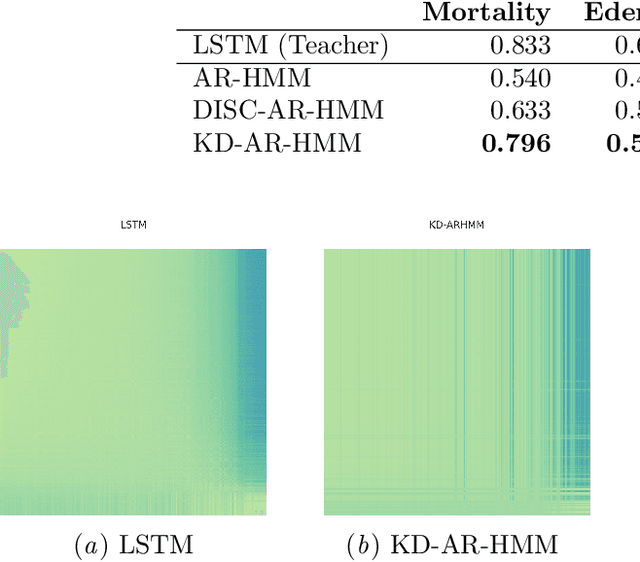

Abstract:Sepsis is a life-threatening condition triggered by an extreme infection response. Our objective is to forecast sepsis patient outcomes using their medical history and treatments, while learning interpretable state representations to assess patients' risks in developing various adverse outcomes. While neural networks excel in outcome prediction, their limited interpretability remains a key issue. In this work, we use knowledge distillation via constrained variational inference to distill the knowledge of a powerful "teacher" neural network model with high predictive power to train a "student" latent variable model to learn interpretable hidden state representations to achieve high predictive performance for sepsis outcome prediction. Using real-world data from the MIMIC-IV database, we trained an LSTM as the "teacher" model to predict mortality for sepsis patients, given information about their recent history of vital signs, lab values and treatments. For our student model, we use an autoregressive hidden Markov model (AR-HMM) to learn interpretable hidden states from patients' clinical time series, and use the posterior distribution of the learned state representations to predict various downstream outcomes, including hospital mortality, pulmonary edema, need for diuretics, dialysis, and mechanical ventilation. Our results show that our approach successfully incorporates the constraint to achieve high predictive power similar to the teacher model, while maintaining the generative performance.
Treatment-RSPN: Recurrent Sum-Product Networks for Sequential Treatment Regimes
Nov 14, 2022Abstract:Sum-product networks (SPNs) have recently emerged as a novel deep learning architecture enabling highly efficient probabilistic inference. Since their introduction, SPNs have been applied to a wide range of data modalities and extended to time-sequence data. In this paper, we propose a general framework for modelling sequential treatment decision-making behaviour and treatment response using recurrent sum-product networks (RSPNs). Models developed using our framework benefit from the full range of RSPN capabilities, including the abilities to model the full distribution of the data, to seamlessly handle latent variables, missing values and categorical data, and to efficiently perform marginal and conditional inference. Our methodology is complemented by a novel variant of the expectation-maximization algorithm for RSPNs, enabling efficient training of our models. We evaluate our approach on a synthetic dataset as well as real-world data from the MIMIC-IV intensive care unit medical database. Our evaluation demonstrates that our approach can closely match the ground-truth data generation process on synthetic data and achieve results close to neural and probabilistic baselines while using a tractable and interpretable model.
Multi-View Spatial-Temporal Graph Convolutional Networks with Domain Generalization for Sleep Stage Classification
Sep 04, 2021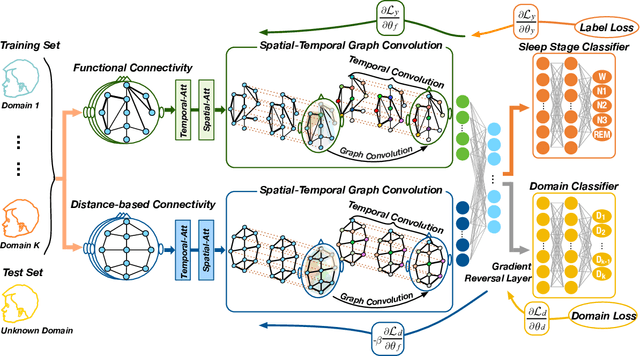
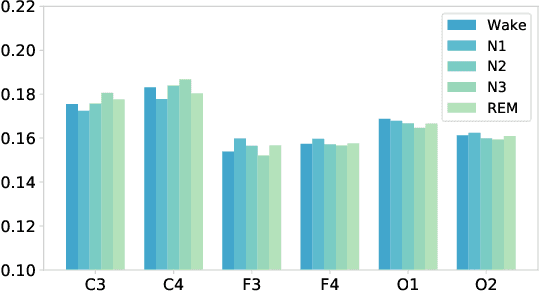
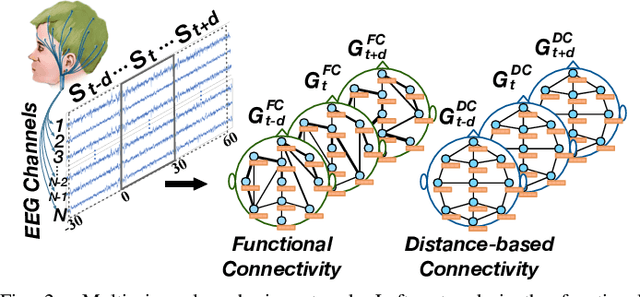
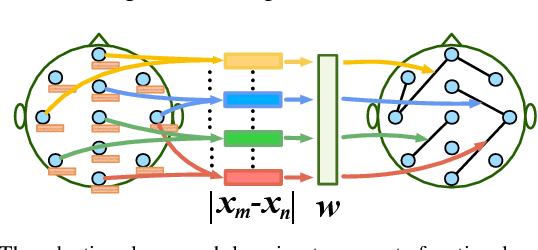
Abstract:Sleep stage classification is essential for sleep assessment and disease diagnosis. Although previous attempts to classify sleep stages have achieved high classification performance, several challenges remain open: 1) How to effectively utilize time-varying spatial and temporal features from multi-channel brain signals remains challenging. Prior works have not been able to fully utilize the spatial topological information among brain regions. 2) Due to the many differences found in individual biological signals, how to overcome the differences of subjects and improve the generalization of deep neural networks is important. 3) Most deep learning methods ignore the interpretability of the model to the brain. To address the above challenges, we propose a multi-view spatial-temporal graph convolutional networks (MSTGCN) with domain generalization for sleep stage classification. Specifically, we construct two brain view graphs for MSTGCN based on the functional connectivity and physical distance proximity of the brain regions. The MSTGCN consists of graph convolutions for extracting spatial features and temporal convolutions for capturing the transition rules among sleep stages. In addition, attention mechanism is employed for capturing the most relevant spatial-temporal information for sleep stage classification. Finally, domain generalization and MSTGCN are integrated into a unified framework to extract subject-invariant sleep features. Experiments on two public datasets demonstrate that the proposed model outperforms the state-of-the-art baselines.
Is Deep Reinforcement Learning Ready for Practical Applications in Healthcare? A Sensitivity Analysis of Duel-DDQN for Sepsis Treatment
May 08, 2020
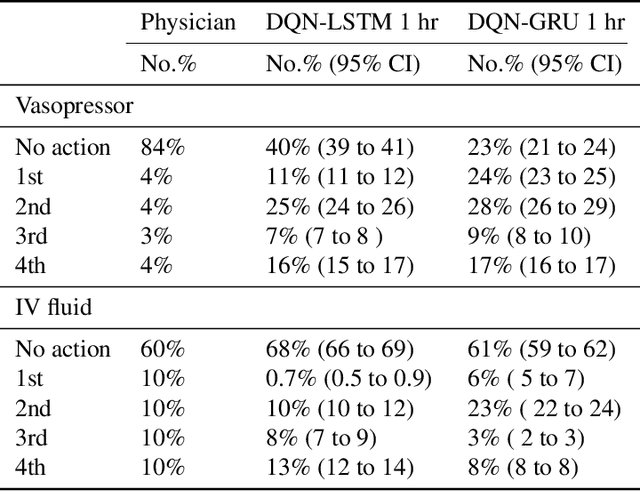
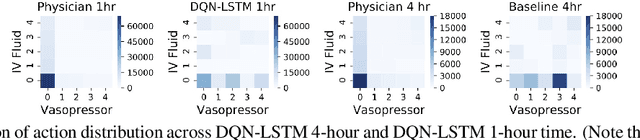
Abstract:The potential of Reinforcement Learning (RL) has been demonstrated through successful applications to games such as Go and Atari. However, while it is straightforward to evaluate the performance of an RL algorithm in a game setting by simply using it to play the game, evaluation is a major challenge in clinical settings where it could be unsafe to follow RL policies in practice. Thus, understanding sensitivity of RL policies to the host of decisions made during implementation is an important step toward building the type of trust in RL required for eventual clinical uptake. In this work, we perform a sensitivity analysis on a state-of-the-art RL algorithm (Dueling Double Deep Q-Networks)applied to hemodynamic stabilization treatment strategies for septic patients in the ICU. We consider sensitivity of learned policies to input features, time discretization, reward function, and random seeds. We find that varying these settings can significantly impact learned policies, which suggests a need for caution when interpreting RL agent output.
G-Net: A Deep Learning Approach to G-computation for Counterfactual Outcome Prediction Under Dynamic Treatment Regimes
Mar 23, 2020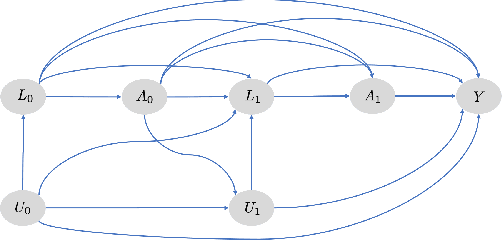
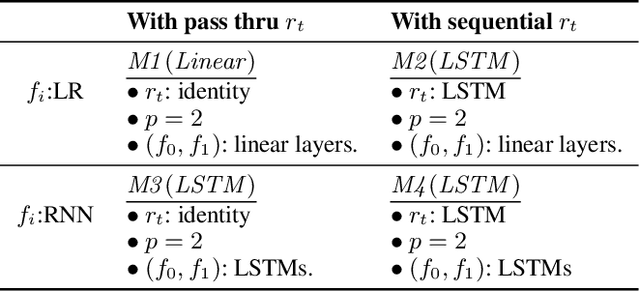
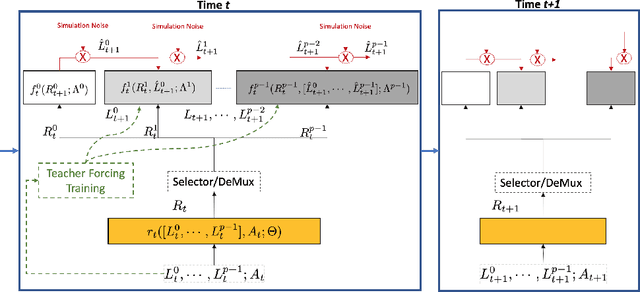
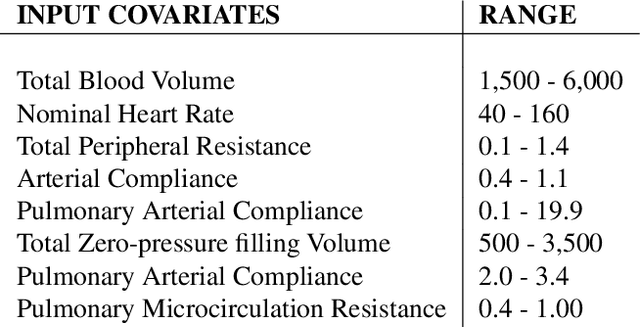
Abstract:Counterfactual prediction is a fundamental task in decision-making. G-computation is a method for estimating expected counterfactual outcomes under dynamic time-varying treatment strategies. Existing G-computation implementations have mostly employed classical regression models with limited capacity to capture complex temporal and nonlinear dependence structures. This paper introduces G-Net, a novel sequential deep learning framework for G-computation that can handle complex time series data while imposing minimal modeling assumptions and provide estimates of individual or population-level time varying treatment effects. We evaluate alternative G-Net implementations using realistically complex temporal simulated data obtained from CVSim, a mechanistic model of the cardiovascular system.
Improving Sepsis Treatment Strategies by Combining Deep and Kernel-Based Reinforcement Learning
Jan 15, 2019
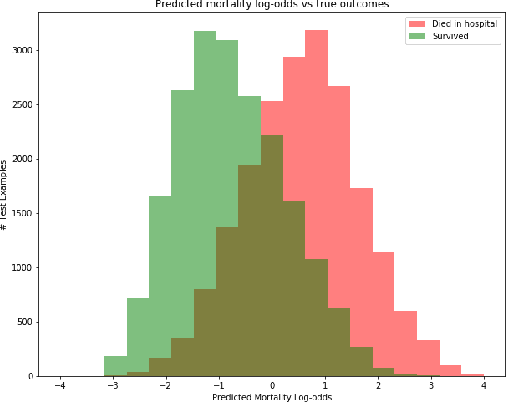

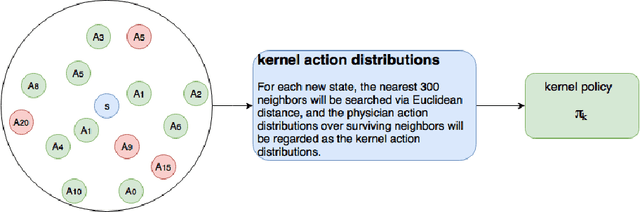
Abstract:Sepsis is the leading cause of mortality in the ICU. It is challenging to manage because individual patients respond differently to treatment. Thus, tailoring treatment to the individual patient is essential for the best outcomes. In this paper, we take steps toward this goal by applying a mixture-of-experts framework to personalize sepsis treatment. The mixture model selectively alternates between neighbor-based (kernel) and deep reinforcement learning (DRL) experts depending on patient's current history. On a large retrospective cohort, this mixture-based approach outperforms physician, kernel only, and DRL-only experts.
Predicting Blood Pressure Response to Fluid Bolus Therapy Using Attention-Based Neural Networks for Clinical Interpretability
Dec 03, 2018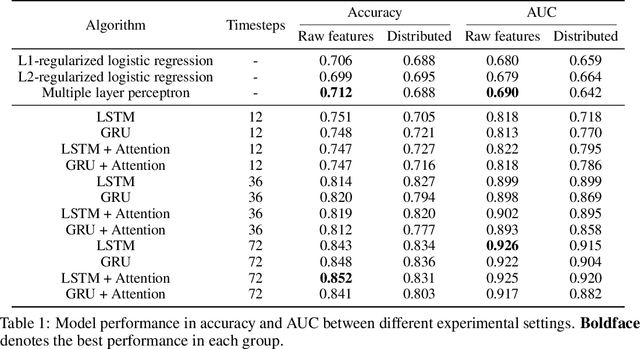
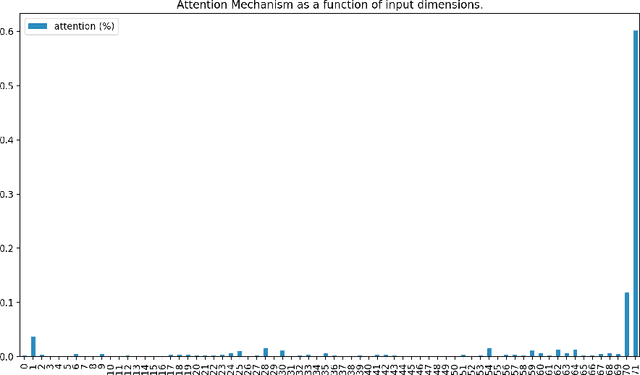
Abstract:Determining whether hypotensive patients in intensive care units (ICUs) should receive fluid bolus therapy (FBT) has been an extremely challenging task for intensive care physicians as the corresponding increase in blood pressure has been hard to predict. Our study utilized regression models and attention-based recurrent neural network (RNN) algorithms and a multi-clinical information system large-scale database to build models that can predict the successful response to FBT among hypotensive patients in ICUs. We investigated both time-aggregated modeling using logistic regression algorithms with regularization and time-series modeling using the long short term memory network (LSTM) and the gated recurrent units network (GRU) with the attention mechanism for clinical interpretability. Among all modeling strategies, the stacked LSTM with the attention mechanism yielded the most predictable model with the highest accuracy of 0.852 and area under the curve (AUC) value of 0.925. The study results may help identify hypotensive patients in ICUs who will have sufficient blood pressure recovery after FBT.
Evaluating Reinforcement Learning Algorithms in Observational Health Settings
May 31, 2018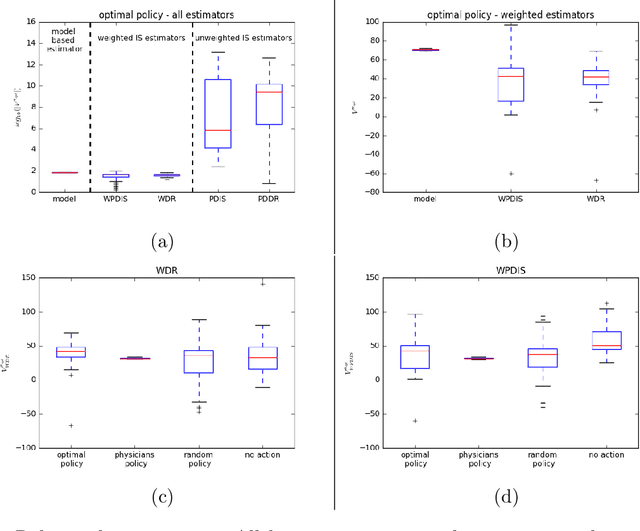
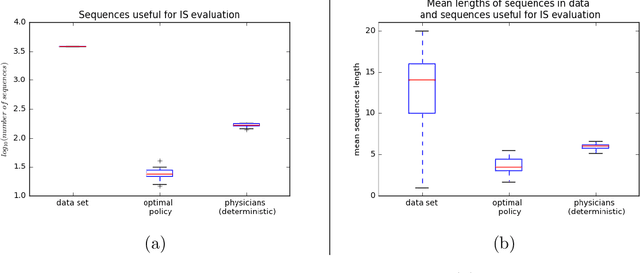
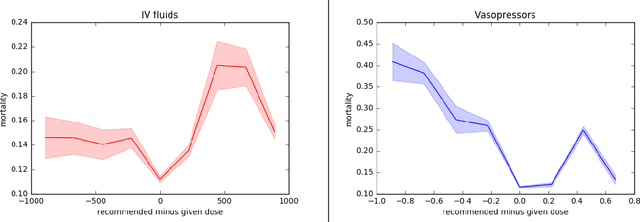
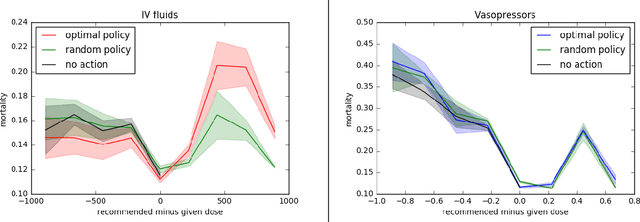
Abstract:Much attention has been devoted recently to the development of machine learning algorithms with the goal of improving treatment policies in healthcare. Reinforcement learning (RL) is a sub-field within machine learning that is concerned with learning how to make sequences of decisions so as to optimize long-term effects. Already, RL algorithms have been proposed to identify decision-making strategies for mechanical ventilation, sepsis management and treatment of schizophrenia. However, before implementing treatment policies learned by black-box algorithms in high-stakes clinical decision problems, special care must be taken in the evaluation of these policies. In this document, our goal is to expose some of the subtleties associated with evaluating RL algorithms in healthcare. We aim to provide a conceptual starting point for clinical and computational researchers to ask the right questions when designing and evaluating algorithms for new ways of treating patients. In the following, we describe how choices about how to summarize a history, variance of statistical estimators, and confounders in more ad-hoc measures can result in unreliable, even misleading estimates of the quality of a treatment policy. We also provide suggestions for mitigating these effects---for while there is much promise for mining observational health data to uncover better treatment policies, evaluation must be performed thoughtfully.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge