Henning Müller
for the Image Biomarker Standardisation Initiative
TransformEEG: Towards Improving Model Generalizability in Deep Learning-based EEG Parkinson's Disease Detection
Jul 10, 2025Abstract:Electroencephalography (EEG) is establishing itself as an important, low-cost, noninvasive diagnostic tool for the early detection of Parkinson's Disease (PD). In this context, EEG-based Deep Learning (DL) models have shown promising results due to their ability to discover highly nonlinear patterns within the signal. However, current state-of-the-art DL models suffer from poor generalizability caused by high inter-subject variability. This high variability underscores the need for enhancing model generalizability by developing new architectures better tailored to EEG data. This paper introduces TransformEEG, a hybrid Convolutional-Transformer designed for Parkinson's disease detection using EEG data. Unlike transformer models based on the EEGNet structure, TransformEEG incorporates a depthwise convolutional tokenizer. This tokenizer is specialized in generating tokens composed by channel-specific features, which enables more effective feature mixing within the self-attention layers of the transformer encoder. To evaluate the proposed model, four public datasets comprising 290 subjects (140 PD patients, 150 healthy controls) were harmonized and aggregated. A 10-outer, 10-inner Nested-Leave-N-Subjects-Out (N-LNSO) cross-validation was performed to provide an unbiased comparison against seven other consolidated EEG deep learning models. TransformEEG achieved the highest balanced accuracy's median (78.45%) as well as the lowest interquartile range (6.37%) across all the N-LNSO partitions. When combined with data augmentation and threshold correction, median accuracy increased to 80.10%, with an interquartile range of 5.74%. In conclusion, TransformEEG produces more consistent and less skewed results. It demonstrates a substantial reduction in variability and more reliable PD detection using EEG data compared to the other investigated models.
A Multi-Centric Anthropomorphic 3D CT Phantom-Based Benchmark Dataset for Harmonization
Jul 02, 2025Abstract:Artificial intelligence (AI) has introduced numerous opportunities for human assistance and task automation in medicine. However, it suffers from poor generalization in the presence of shifts in the data distribution. In the context of AI-based computed tomography (CT) analysis, significant data distribution shifts can be caused by changes in scanner manufacturer, reconstruction technique or dose. AI harmonization techniques can address this problem by reducing distribution shifts caused by various acquisition settings. This paper presents an open-source benchmark dataset containing CT scans of an anthropomorphic phantom acquired with various scanners and settings, which purpose is to foster the development of AI harmonization techniques. Using a phantom allows fixing variations attributed to inter- and intra-patient variations. The dataset includes 1378 image series acquired with 13 scanners from 4 manufacturers across 8 institutions using a harmonized protocol as well as several acquisition doses. Additionally, we present a methodology, baseline results and open-source code to assess image- and feature-level stability and liver tissue classification, promoting the development of AI harmonization strategies.
Explainability of AI Uncertainty: Application to Multiple Sclerosis Lesion Segmentation on MRI
Apr 07, 2025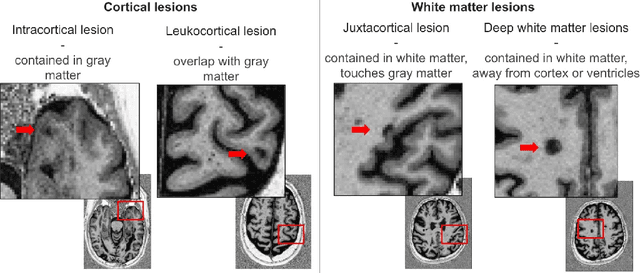



Abstract:Trustworthy artificial intelligence (AI) is essential in healthcare, particularly for high-stakes tasks like medical image segmentation. Explainable AI and uncertainty quantification significantly enhance AI reliability by addressing key attributes such as robustness, usability, and explainability. Despite extensive technical advances in uncertainty quantification for medical imaging, understanding the clinical informativeness and interpretability of uncertainty remains limited. This study introduces a novel framework to explain the potential sources of predictive uncertainty, specifically in cortical lesion segmentation in multiple sclerosis using deep ensembles. The proposed analysis shifts the focus from the uncertainty-error relationship towards relevant medical and engineering factors. Our findings reveal that instance-wise uncertainty is strongly related to lesion size, shape, and cortical involvement. Expert rater feedback confirms that similar factors impede annotator confidence. Evaluations conducted on two datasets (206 patients, almost 2000 lesions) under both in-domain and distribution-shift conditions highlight the utility of the framework in different scenarios.
3-D Image-to-Image Fusion in Lightsheet Microscopy by Two-Step Adversarial Network: Contribution to the FuseMyCells Challenge
Mar 20, 2025Abstract:Lightsheet microscopy is a powerful 3-D imaging technique that addresses limitations of traditional optical and confocal microscopy but suffers from a low penetration depth and reduced image quality at greater depths. Multiview lightsheet microscopy improves 3-D resolution by combining multiple views but simultaneously increasing the complexity and the photon budget, leading to potential photobleaching and phototoxicity. The FuseMyCells challenge, organized in conjunction with the IEEE ISBI 2025 conference, aims to benchmark deep learning-based solutions for fusing high-quality 3-D volumes from single 3-D views, potentially simplifying procedures and conserving the photon budget. In this work, we propose a contribution to the FuseMyCells challenge based on a two-step procedure. The first step processes a downsampled version of the image to capture the entire region of interest, while the second step uses a patch-based approach for high-resolution inference, incorporating adversarial loss to enhance visual outcomes. This method addresses challenges related to high data resolution, the necessity of global context, and the preservation of high-frequency details. Experimental results demonstrate the effectiveness of our approach, highlighting its potential to improve 3-D image fusion quality and extend the capabilities of lightsheet microscopy. The average SSIM for the nucleus and membranes is greater than 0.85 and 0.91, respectively.
Automatic Registration of SHG and H&E Images with Feature-based Initial Alignment and Intensity-based Instance Optimization: Contribution to the COMULIS Challenge
Sep 24, 2024Abstract:The automatic registration of noninvasive second-harmonic generation microscopy to hematoxylin and eosin slides is a highly desired, yet still unsolved problem. The task is challenging because the second-harmonic images contain only partial information, in contrast to the stained H&E slides that provide more information about the tissue morphology. Moreover, both imaging methods have different intensity distributions. Therefore, the task can be formulated as a multi-modal registration problem with missing data. In this work, we propose a method based on automatic keypoint matching followed by deformable registration based on instance optimization. The method does not require any training and is evaluated using the dataset provided in the Learn2Reg challenge by the COMULIS organization. The method achieved relatively good generalizability resulting in 88% of success rate in the initial alignment and average target registration error equal to 2.48 on the external validation set. We openly release the source code and incorporate it in the DeeperHistReg image registration framework.
Lymphoid Infiltration Assessment of the Tumor Margins in H&E Slides
Jul 23, 2024



Abstract:Lymphoid infiltration at tumor margins is a key prognostic marker in solid tumors, playing a crucial role in guiding immunotherapy decisions. Current assessment methods, heavily reliant on immunohistochemistry (IHC), face challenges in tumor margin delineation and are affected by tissue preservation conditions. In contrast, we propose a Hematoxylin and Eosin (H&E) staining-based approach, underpinned by an advanced lymphocyte segmentation model trained on a public dataset for the precise detection of CD3+ and CD20+ lymphocytes. In our colorectal cancer study, we demonstrate that our H&E-based method offers a compelling alternative to traditional IHC, achieving comparable results in many cases. Our method's validity is further explored through a Turing test, involving blinded assessments by a pathologist of anonymized curves from H&E and IHC slides. This approach invites the medical community to consider Turing tests as a standard for evaluating medical applications involving expert human evaluation, thereby opening new avenues for enhancing cancer management and immunotherapy planning.
Interpretability of Uncertainty: Exploring Cortical Lesion Segmentation in Multiple Sclerosis
Jul 08, 2024


Abstract:Uncertainty quantification (UQ) has become critical for evaluating the reliability of artificial intelligence systems, especially in medical image segmentation. This study addresses the interpretability of instance-wise uncertainty values in deep learning models for focal lesion segmentation in magnetic resonance imaging, specifically cortical lesion (CL) segmentation in multiple sclerosis. CL segmentation presents several challenges, including the complexity of manual segmentation, high variability in annotation, data scarcity, and class imbalance, all of which contribute to aleatoric and epistemic uncertainty. We explore how UQ can be used not only to assess prediction reliability but also to provide insights into model behavior, detect biases, and verify the accuracy of UQ methods. Our research demonstrates the potential of instance-wise uncertainty values to offer post hoc global model explanations, serving as a sanity check for the model. The implementation is available at https://github.com/NataliiaMolch/interpret-lesion-unc.
Automatic Labels are as Effective as Manual Labels in Biomedical Images Classification with Deep Learning
Jun 20, 2024Abstract:The increasing availability of biomedical data is helping to design more robust deep learning (DL) algorithms to analyze biomedical samples. Currently, one of the main limitations to train DL algorithms to perform a specific task is the need for medical experts to label data. Automatic methods to label data exist, however automatic labels can be noisy and it is not completely clear when automatic labels can be adopted to train DL models. This paper aims to investigate under which circumstances automatic labels can be adopted to train a DL model on the classification of Whole Slide Images (WSI). The analysis involves multiple architectures, such as Convolutional Neural Networks (CNN) and Vision Transformer (ViT), and over 10000 WSIs, collected from three use cases: celiac disease, lung cancer and colon cancer, which one including respectively binary, multiclass and multilabel data. The results allow identifying 10% as the percentage of noisy labels that lead to train competitive models for the classification of WSIs. Therefore, an algorithm generating automatic labels needs to fit this criterion to be adopted. The application of the Semantic Knowledge Extractor Tool (SKET) algorithm to generate automatic labels leads to performance comparable to the one obtained with manual labels, since it generates a percentage of noisy labels between 2-5%. Automatic labels are as effective as manual ones, reaching solid performance comparable to the one obtained training models with manual labels.
Improving Quality Control of Whole Slide Images by Explicit Artifact Augmentation
Jun 17, 2024Abstract:The problem of artifacts in whole slide image acquisition, prevalent in both clinical workflows and research-oriented settings, necessitates human intervention and re-scanning. Overcoming this challenge requires developing quality control algorithms, that are hindered by the limited availability of relevant annotated data in histopathology. The manual annotation of ground-truth for artifact detection methods is expensive and time-consuming. This work addresses the issue by proposing a method dedicated to augmenting whole slide images with artifacts. The tool seamlessly generates and blends artifacts from an external library to a given histopathology dataset. The augmented datasets are then utilized to train artifact classification methods. The evaluation shows their usefulness in classification of the artifacts, where they show an improvement from 0.10 to 0.01 AUROC depending on the artifact type. The framework, model, weights, and ground-truth annotations are freely released to facilitate open science and reproducible research.
Patch-Based Encoder-Decoder Architecture for Automatic Transmitted Light to Fluorescence Imaging Transition: Contribution to the LightMyCells Challenge
Jun 03, 2024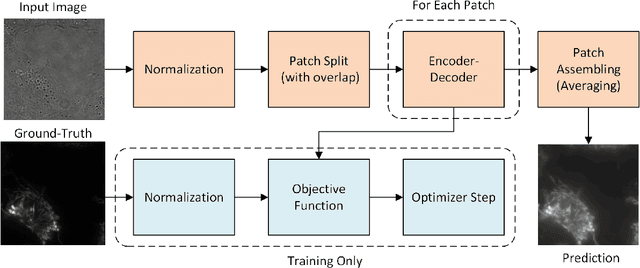
Abstract:Automatic prediction of fluorescently labeled organelles from label-free transmitted light input images is an important, yet difficult task. The traditional way to obtain fluorescence images is related to performing biochemical labeling which is time-consuming and costly. Therefore, an automatic algorithm to perform the task based on the label-free transmitted light microscopy could be strongly beneficial. The importance of the task motivated researchers from the France-BioImaging to organize the LightMyCells challenge where the goal is to propose an algorithm that automatically predicts the fluorescently labeled nucleus, mitochondria, tubulin, and actin, based on the input consisting of bright field, phase contrast, or differential interference contrast microscopic images. In this work, we present the contribution of the AGHSSO team based on a carefully prepared and trained encoder-decoder deep neural network that achieves a considerable score in the challenge, being placed among the best-performing teams.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge