Cynthia S. Schmidt
A Multimodal Pipeline for Clinical Data Extraction: Applying Vision-Language Models to Scans of Transfusion Reaction Reports
Apr 28, 2025Abstract:Despite the growing adoption of electronic health records, many processes still rely on paper documents, reflecting the heterogeneous real-world conditions in which healthcare is delivered. The manual transcription process is time-consuming and prone to errors when transferring paper-based data to digital formats. To streamline this workflow, this study presents an open-source pipeline that extracts and categorizes checkbox data from scanned documents. Demonstrated on transfusion reaction reports, the design supports adaptation to other checkbox-rich document types. The proposed method integrates checkbox detection, multilingual optical character recognition (OCR) and multilingual vision-language models (VLMs). The pipeline achieves high precision and recall compared against annually compiled gold-standards from 2017 to 2024. The result is a reduction in administrative workload and accurate regulatory reporting. The open-source availability of this pipeline encourages self-hosted parsing of checkbox forms.
SALT: Introducing a Framework for Hierarchical Segmentations in Medical Imaging using Softmax for Arbitrary Label Trees
Jul 11, 2024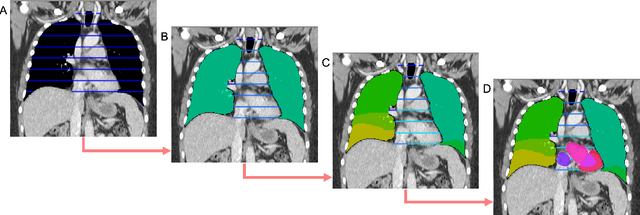
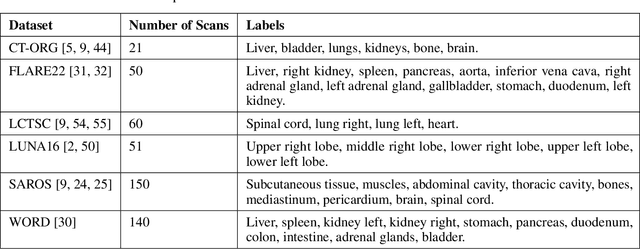
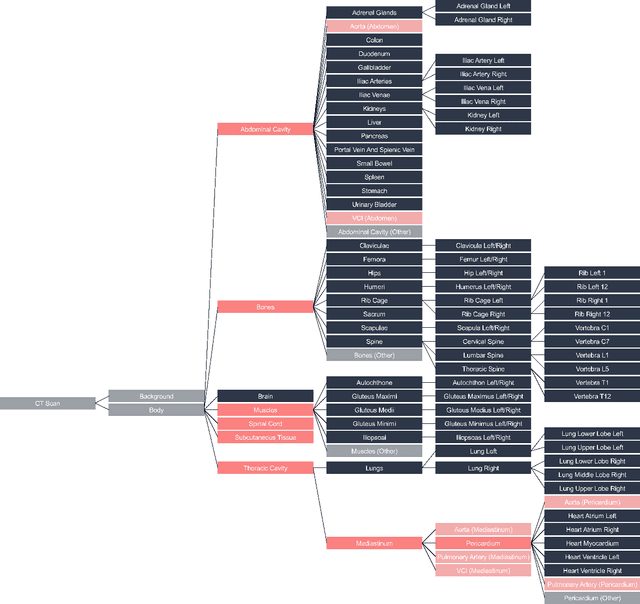
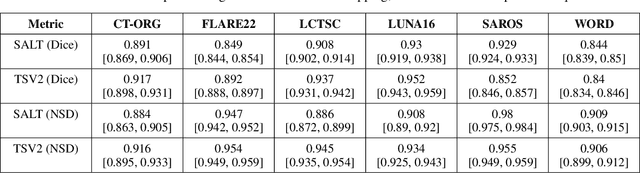
Abstract:Traditional segmentation networks approach anatomical structures as standalone elements, overlooking the intrinsic hierarchical connections among them. This study introduces Softmax for Arbitrary Label Trees (SALT), a novel approach designed to leverage the hierarchical relationships between labels, improving the efficiency and interpretability of the segmentations. This study introduces a novel segmentation technique for CT imaging, which leverages conditional probabilities to map the hierarchical structure of anatomical landmarks, such as the spine's division into lumbar, thoracic, and cervical regions and further into individual vertebrae. The model was developed using the SAROS dataset from The Cancer Imaging Archive (TCIA), comprising 900 body region segmentations from 883 patients. The dataset was further enhanced by generating additional segmentations with the TotalSegmentator, for a total of 113 labels. The model was trained on 600 scans, while validation and testing were conducted on 150 CT scans. Performance was assessed using the Dice score across various datasets, including SAROS, CT-ORG, FLARE22, LCTSC, LUNA16, and WORD. Among the evaluated datasets, SALT achieved its best results on the LUNA16 and SAROS datasets, with Dice scores of 0.93 and 0.929 respectively. The model demonstrated reliable accuracy across other datasets, scoring 0.891 on CT-ORG and 0.849 on FLARE22. The LCTSC dataset showed a score of 0.908 and the WORD dataset also showed good performance with a score of 0.844. SALT used the hierarchical structures inherent in the human body to achieve whole-body segmentations with an average of 35 seconds for 100 slices. This rapid processing underscores its potential for integration into clinical workflows, facilitating the automatic and efficient computation of full-body segmentations with each CT scan, thus enhancing diagnostic processes and patient care.
ROCOv2: Radiology Objects in COntext Version 2, an Updated Multimodal Image Dataset
May 16, 2024Abstract:Automated medical image analysis systems often require large amounts of training data with high quality labels, which are difficult and time consuming to generate. This paper introduces Radiology Object in COntext version 2 (ROCOv2), a multimodal dataset consisting of radiological images and associated medical concepts and captions extracted from the PMC Open Access subset. It is an updated version of the ROCO dataset published in 2018, and adds 35,705 new images added to PMC since 2018. It further provides manually curated concepts for imaging modalities with additional anatomical and directional concepts for X-rays. The dataset consists of 79,789 images and has been used, with minor modifications, in the concept detection and caption prediction tasks of ImageCLEFmedical Caption 2023. The dataset is suitable for training image annotation models based on image-caption pairs, or for multi-label image classification using Unified Medical Language System (UMLS) concepts provided with each image. In addition, it can serve for pre-training of medical domain models, and evaluation of deep learning models for multi-task learning.
Comprehensive Study on German Language Models for Clinical and Biomedical Text Understanding
Apr 08, 2024



Abstract:Recent advances in natural language processing (NLP) can be largely attributed to the advent of pre-trained language models such as BERT and RoBERTa. While these models demonstrate remarkable performance on general datasets, they can struggle in specialized domains such as medicine, where unique domain-specific terminologies, domain-specific abbreviations, and varying document structures are common. This paper explores strategies for adapting these models to domain-specific requirements, primarily through continuous pre-training on domain-specific data. We pre-trained several German medical language models on 2.4B tokens derived from translated public English medical data and 3B tokens of German clinical data. The resulting models were evaluated on various German downstream tasks, including named entity recognition (NER), multi-label classification, and extractive question answering. Our results suggest that models augmented by clinical and translation-based pre-training typically outperform general domain models in medical contexts. We conclude that continuous pre-training has demonstrated the ability to match or even exceed the performance of clinical models trained from scratch. Furthermore, pre-training on clinical data or leveraging translated texts have proven to be reliable methods for domain adaptation in medical NLP tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge