Andrew Zhang
SteerVLM: Robust Model Control through Lightweight Activation Steering for Vision Language Models
Oct 30, 2025Abstract:This work introduces SteerVLM, a lightweight steering module designed to guide Vision-Language Models (VLMs) towards outputs that better adhere to desired instructions. Our approach learns from the latent embeddings of paired prompts encoding target and converse behaviors to dynamically adjust activations connecting the language modality with image context. This allows for fine-grained, inference-time control over complex output semantics without modifying model weights while preserving performance on off-target tasks. Our steering module requires learning parameters equal to 0.14% of the original VLM's size. Our steering module gains model control through dimension-wise activation modulation and adaptive steering across layers without requiring pre-extracted static vectors or manual tuning of intervention points. Furthermore, we introduce VNIA (Visual Narrative Intent Alignment), a multimodal dataset specifically created to facilitate the development and evaluation of VLM steering techniques. Our method outperforms existing intervention techniques on steering and hallucination mitigation benchmarks for VLMs and proposes a robust solution for multimodal model control through activation engineering.
Maximal Matching Matters: Preventing Representation Collapse for Robust Cross-Modal Retrieval
Jun 26, 2025
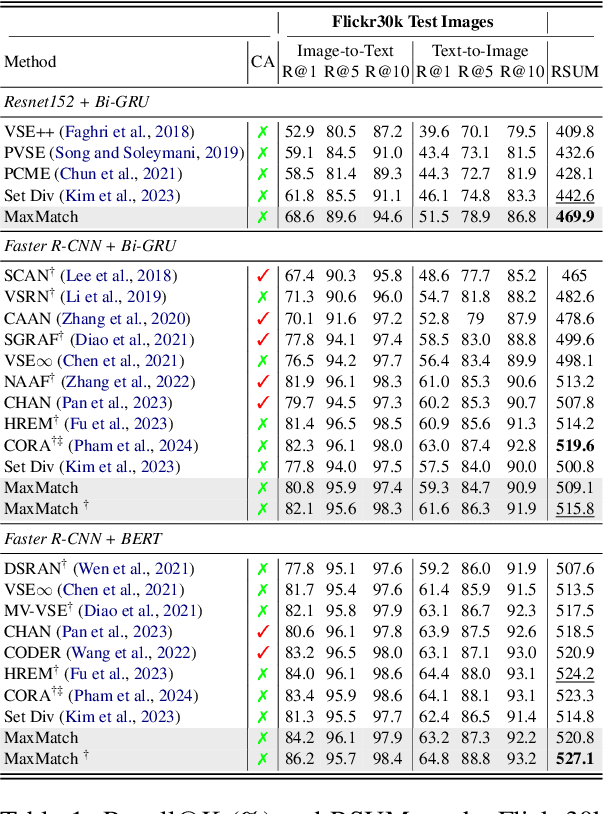


Abstract:Cross-modal image-text retrieval is challenging because of the diverse possible associations between content from different modalities. Traditional methods learn a single-vector embedding to represent semantics of each sample, but struggle to capture nuanced and diverse relationships that can exist across modalities. Set-based approaches, which represent each sample with multiple embeddings, offer a promising alternative, as they can capture richer and more diverse relationships. In this paper, we show that, despite their promise, these set-based representations continue to face issues including sparse supervision and set collapse, which limits their effectiveness. To address these challenges, we propose Maximal Pair Assignment Similarity to optimize one-to-one matching between embedding sets which preserve semantic diversity within the set. We also introduce two loss functions to further enhance the representations: Global Discriminative Loss to enhance distinction among embeddings, and Intra-Set Divergence Loss to prevent collapse within each set. Our method achieves state-of-the-art performance on MS-COCO and Flickr30k without relying on external data.
Flexible-length Text Infilling for Discrete Diffusion Models
Jun 16, 2025
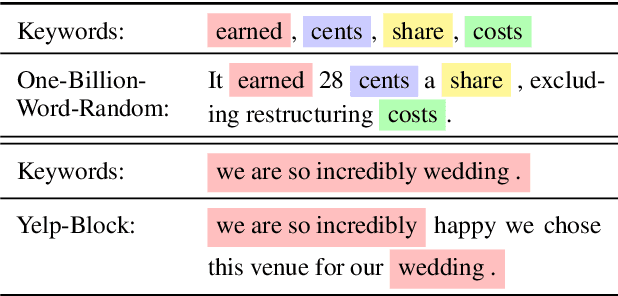
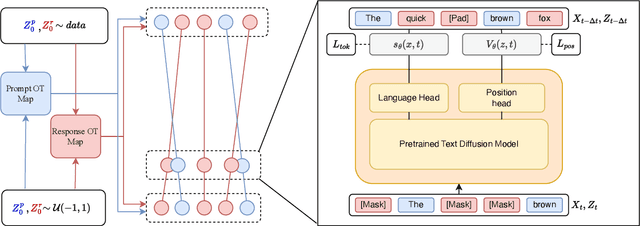

Abstract:Discrete diffusion models are a new class of text generators that offer advantages such as bidirectional context use, parallelizable generation, and flexible prompting compared to autoregressive models. However, a critical limitation of discrete diffusion models is their inability to perform flexible-length or flexible-position text infilling without access to ground-truth positional data. We introduce \textbf{DDOT} (\textbf{D}iscrete \textbf{D}iffusion with \textbf{O}ptimal \textbf{T}ransport Position Coupling), the first discrete diffusion model to overcome this challenge. DDOT jointly denoises token values and token positions, employing a novel sample-level Optimal Transport (OT) coupling. This coupling preserves relative token ordering while dynamically adjusting the positions and length of infilled segments, a capability previously missing in text diffusion. Our method is orthogonal to existing discrete text diffusion methods and is compatible with various pretrained text denoisers. Extensive experiments on text infilling benchmarks such as One-Billion-Word and Yelp demonstrate that DDOT outperforms naive diffusion baselines. Furthermore, DDOT achieves performance on par with state-of-the-art non-autoregressive models and enables significant improvements in training efficiency and flexibility.
MAISY: Motion-Aware Image SYnthesis for Medical Image Motion Correction
May 08, 2025Abstract:Patient motion during medical image acquisition causes blurring, ghosting, and distorts organs, which makes image interpretation challenging. Current state-of-the-art algorithms using Generative Adversarial Network (GAN)-based methods with their ability to learn the mappings between corrupted images and their ground truth via Structural Similarity Index Measure (SSIM) loss effectively generate motion-free images. However, we identified the following limitations: (i) they mainly focus on global structural characteristics and therefore overlook localized features that often carry critical pathological information, and (ii) the SSIM loss function struggles to handle images with varying pixel intensities, luminance factors, and variance. In this study, we propose Motion-Aware Image SYnthesis (MAISY) which initially characterize motion and then uses it for correction by: (a) leveraging the foundation model Segment Anything Model (SAM), to dynamically learn spatial patterns along anatomical boundaries where motion artifacts are most pronounced and, (b) introducing the Variance-Selective SSIM (VS-SSIM) loss which adaptively emphasizes spatial regions with high pixel variance to preserve essential anatomical details during artifact correction. Experiments on chest and head CT datasets demonstrate that our model outperformed the state-of-the-art counterparts, with Peak Signal-to-Noise Ratio (PSNR) increasing by 40%, SSIM by 10%, and Dice by 16%.
Accelerating Data Processing and Benchmarking of AI Models for Pathology
Feb 10, 2025Abstract:Advances in foundation modeling have reshaped computational pathology. However, the increasing number of available models and lack of standardized benchmarks make it increasingly complex to assess their strengths, limitations, and potential for further development. To address these challenges, we introduce a new suite of software tools for whole-slide image processing, foundation model benchmarking, and curated publicly available tasks. We anticipate that these resources will promote transparency, reproducibility, and continued progress in the field.
Molecular-driven Foundation Model for Oncologic Pathology
Jan 28, 2025Abstract:Foundation models are reshaping computational pathology by enabling transfer learning, where models pre-trained on vast datasets can be adapted for downstream diagnostic, prognostic, and therapeutic response tasks. Despite these advances, foundation models are still limited in their ability to encode the entire gigapixel whole-slide images without additional training and often lack complementary multimodal data. Here, we introduce Threads, a slide-level foundation model capable of generating universal representations of whole-slide images of any size. Threads was pre-trained using a multimodal learning approach on a diverse cohort of 47,171 hematoxylin and eosin (H&E)-stained tissue sections, paired with corresponding genomic and transcriptomic profiles - the largest such paired dataset to be used for foundation model development to date. This unique training paradigm enables Threads to capture the tissue's underlying molecular composition, yielding powerful representations applicable to a wide array of downstream tasks. In extensive benchmarking across 54 oncology tasks, including clinical subtyping, grading, mutation prediction, immunohistochemistry status determination, treatment response prediction, and survival prediction, Threads outperformed all baselines while demonstrating remarkable generalizability and label efficiency. It is particularly well suited for predicting rare events, further emphasizing its clinical utility. We intend to make the model publicly available for the broader community.
Multimodal Whole Slide Foundation Model for Pathology
Nov 29, 2024



Abstract:The field of computational pathology has been transformed with recent advances in foundation models that encode histopathology region-of-interests (ROIs) into versatile and transferable feature representations via self-supervised learning (SSL). However, translating these advancements to address complex clinical challenges at the patient and slide level remains constrained by limited clinical data in disease-specific cohorts, especially for rare clinical conditions. We propose TITAN, a multimodal whole slide foundation model pretrained using 335,645 WSIs via visual self-supervised learning and vision-language alignment with corresponding pathology reports and 423,122 synthetic captions generated from a multimodal generative AI copilot for pathology. Without any finetuning or requiring clinical labels, TITAN can extract general-purpose slide representations and generate pathology reports that generalize to resource-limited clinical scenarios such as rare disease retrieval and cancer prognosis. We evaluate TITAN on diverse clinical tasks and find that TITAN outperforms both ROI and slide foundation models across machine learning settings such as linear probing, few-shot and zero-shot classification, rare cancer retrieval and cross-modal retrieval, and pathology report generation.
Multistain Pretraining for Slide Representation Learning in Pathology
Aug 05, 2024Abstract:Developing self-supervised learning (SSL) models that can learn universal and transferable representations of H&E gigapixel whole-slide images (WSIs) is becoming increasingly valuable in computational pathology. These models hold the potential to advance critical tasks such as few-shot classification, slide retrieval, and patient stratification. Existing approaches for slide representation learning extend the principles of SSL from small images (e.g., 224 x 224 patches) to entire slides, usually by aligning two different augmentations (or views) of the slide. Yet the resulting representation remains constrained by the limited clinical and biological diversity of the views. Instead, we postulate that slides stained with multiple markers, such as immunohistochemistry, can be used as different views to form a rich task-agnostic training signal. To this end, we introduce Madeleine, a multimodal pretraining strategy for slide representation learning. Madeleine is trained with a dual global-local cross-stain alignment objective on large cohorts of breast cancer samples (N=4,211 WSIs across five stains) and kidney transplant samples (N=12,070 WSIs across four stains). We demonstrate the quality of slide representations learned by Madeleine on various downstream evaluations, ranging from morphological and molecular classification to prognostic prediction, comprising 21 tasks using 7,299 WSIs from multiple medical centers. Code is available at https://github.com/mahmoodlab/MADELEINE.
MedCalc-Bench: Evaluating Large Language Models for Medical Calculations
Jun 17, 2024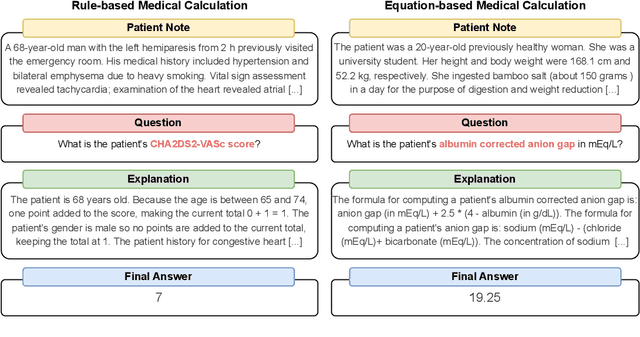
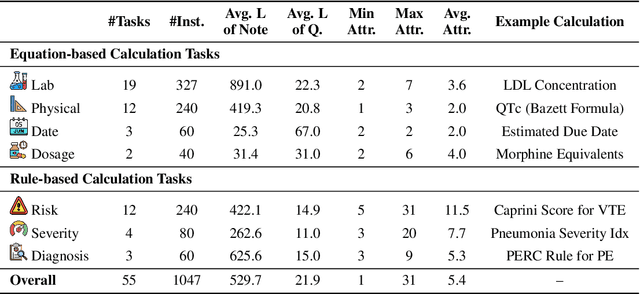
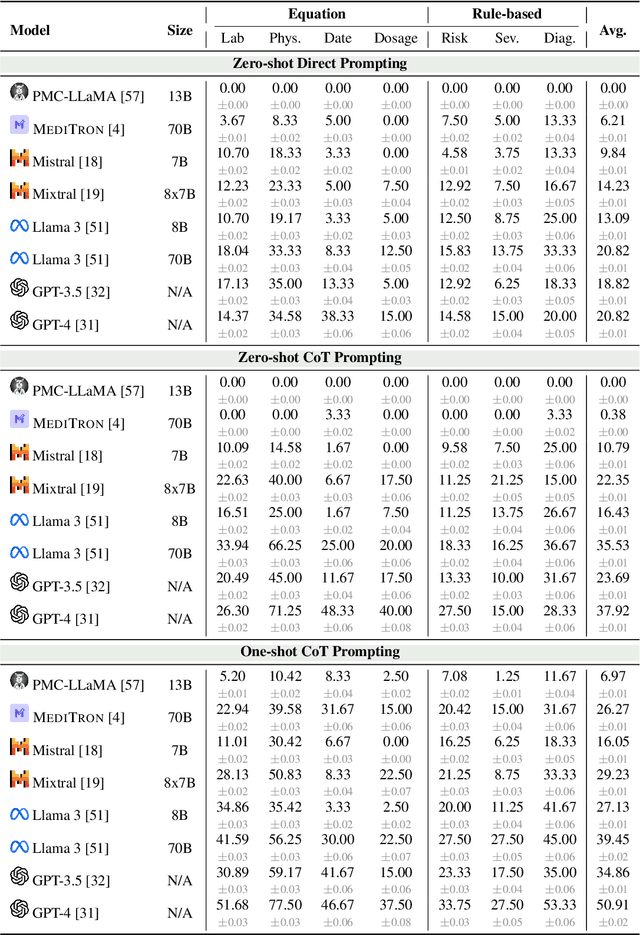
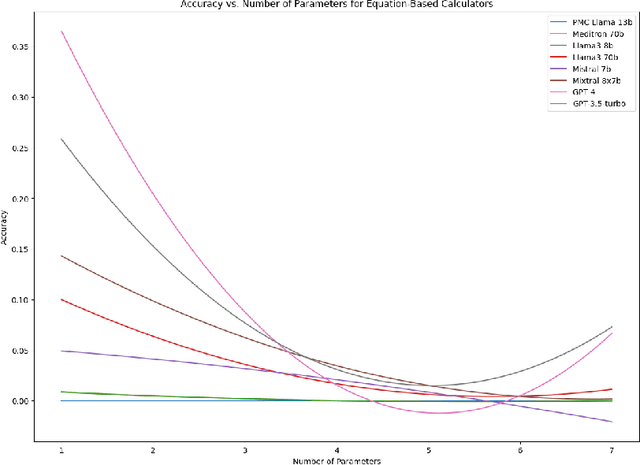
Abstract:As opposed to evaluating computation and logic-based reasoning, current bench2 marks for evaluating large language models (LLMs) in medicine are primarily focused on question-answering involving domain knowledge and descriptive rea4 soning. While such qualitative capabilities are vital to medical diagnosis, in real5 world scenarios, doctors frequently use clinical calculators that follow quantitative equations and rule-based reasoning paradigms for evidence-based decision support. To this end, we propose MedCalc-Bench, a first-of-its-kind dataset focused on evaluating the medical calculation capability of LLMs. MedCalc-Bench contains an evaluation set of over 1000 manually reviewed instances from 55 different medical calculation tasks. Each instance in MedCalc-Bench consists of a patient note, a question requesting to compute a specific medical value, a ground truth answer, and a step-by-step explanation showing how the answer is obtained. While our evaluation results show the potential of LLMs in this area, none of them are effective enough for clinical settings. Common issues include extracting the incorrect entities, not using the correct equation or rules for a calculation task, or incorrectly performing the arithmetic for the computation. We hope our study highlights the quantitative knowledge and reasoning gaps in LLMs within medical settings, encouraging future improvements of LLMs for various clinical calculation tasks.
A General-Purpose Self-Supervised Model for Computational Pathology
Aug 29, 2023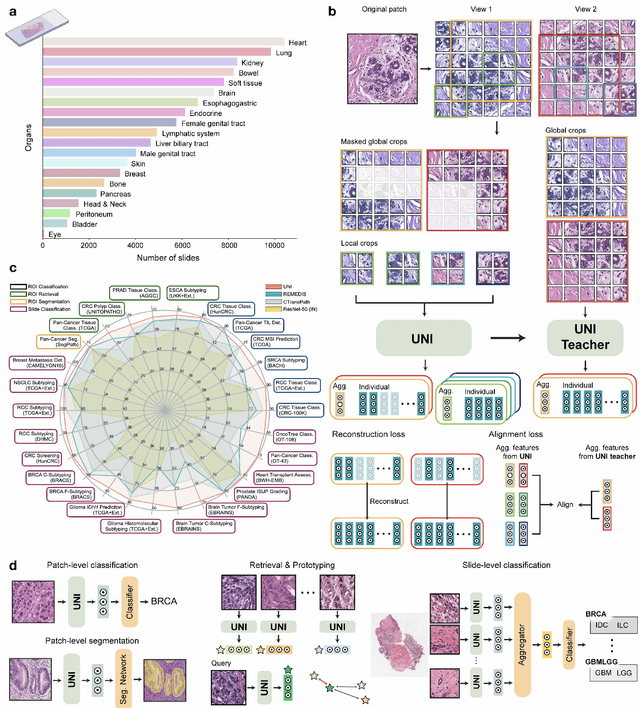

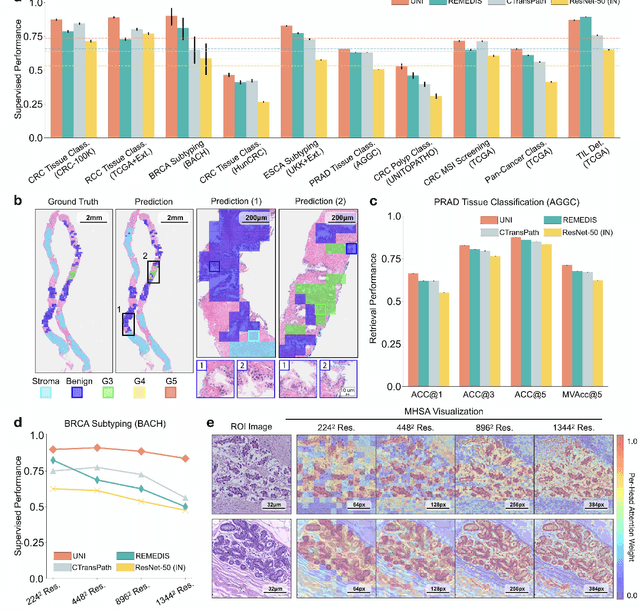

Abstract:Tissue phenotyping is a fundamental computational pathology (CPath) task in learning objective characterizations of histopathologic biomarkers in anatomic pathology. However, whole-slide imaging (WSI) poses a complex computer vision problem in which the large-scale image resolutions of WSIs and the enormous diversity of morphological phenotypes preclude large-scale data annotation. Current efforts have proposed using pretrained image encoders with either transfer learning from natural image datasets or self-supervised pretraining on publicly-available histopathology datasets, but have not been extensively developed and evaluated across diverse tissue types at scale. We introduce UNI, a general-purpose self-supervised model for pathology, pretrained using over 100 million tissue patches from over 100,000 diagnostic haematoxylin and eosin-stained WSIs across 20 major tissue types, and evaluated on 33 representative CPath clinical tasks in CPath of varying diagnostic difficulties. In addition to outperforming previous state-of-the-art models, we demonstrate new modeling capabilities in CPath such as resolution-agnostic tissue classification, slide classification using few-shot class prototypes, and disease subtyping generalization in classifying up to 108 cancer types in the OncoTree code classification system. UNI advances unsupervised representation learning at scale in CPath in terms of both pretraining data and downstream evaluation, enabling data-efficient AI models that can generalize and transfer to a gamut of diagnostically-challenging tasks and clinical workflows in anatomic pathology.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge