Guillaume Jaume
Evidence-based diagnostic reasoning with multi-agent copilot for human pathology
Jun 26, 2025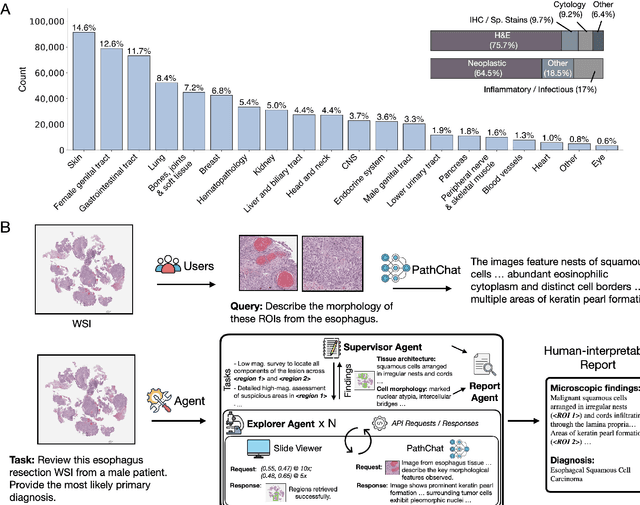
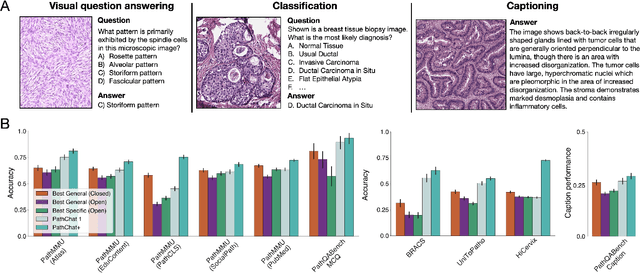
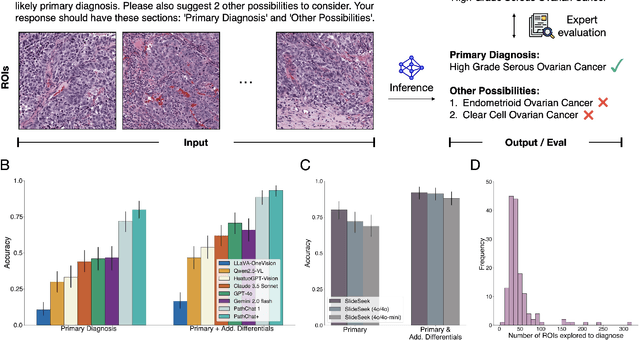
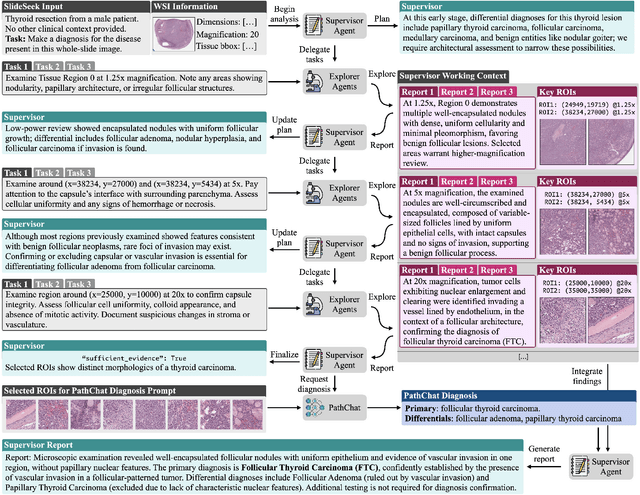
Abstract:Pathology is experiencing rapid digital transformation driven by whole-slide imaging and artificial intelligence (AI). While deep learning-based computational pathology has achieved notable success, traditional models primarily focus on image analysis without integrating natural language instruction or rich, text-based context. Current multimodal large language models (MLLMs) in computational pathology face limitations, including insufficient training data, inadequate support and evaluation for multi-image understanding, and a lack of autonomous, diagnostic reasoning capabilities. To address these limitations, we introduce PathChat+, a new MLLM specifically designed for human pathology, trained on over 1 million diverse, pathology-specific instruction samples and nearly 5.5 million question answer turns. Extensive evaluations across diverse pathology benchmarks demonstrated that PathChat+ substantially outperforms the prior PathChat copilot, as well as both state-of-the-art (SOTA) general-purpose and other pathology-specific models. Furthermore, we present SlideSeek, a reasoning-enabled multi-agent AI system leveraging PathChat+ to autonomously evaluate gigapixel whole-slide images (WSIs) through iterative, hierarchical diagnostic reasoning, reaching high accuracy on DDxBench, a challenging open-ended differential diagnosis benchmark, while also capable of generating visually grounded, humanly-interpretable summary reports.
AI-driven 3D Spatial Transcriptomics
Feb 25, 2025



Abstract:A comprehensive three-dimensional (3D) map of tissue architecture and gene expression is crucial for illuminating the complexity and heterogeneity of tissues across diverse biomedical applications. However, most spatial transcriptomics (ST) approaches remain limited to two-dimensional (2D) sections of tissue. Although current 3D ST methods hold promise, they typically require extensive tissue sectioning, are complex, are not compatible with non-destructive 3D tissue imaging technologies, and often lack scalability. Here, we present VOlumetrically Resolved Transcriptomics EXpression (VORTEX), an AI framework that leverages 3D tissue morphology and minimal 2D ST to predict volumetric 3D ST. By pretraining on diverse 3D morphology-transcriptomic pairs from heterogeneous tissue samples and then fine-tuning on minimal 2D ST data from a specific volume of interest, VORTEX learns both generic tissue-related and sample-specific morphological correlates of gene expression. This approach enables dense, high-throughput, and fast 3D ST, scaling seamlessly to large tissue volumes far beyond the reach of existing 3D ST techniques. By offering a cost-effective and minimally destructive route to obtaining volumetric molecular insights, we anticipate that VORTEX will accelerate biomarker discovery and our understanding of morphomolecular associations and cell states in complex tissues. Interactive 3D ST volumes can be viewed at https://vortex-demo.github.io/
Accelerating Data Processing and Benchmarking of AI Models for Pathology
Feb 10, 2025Abstract:Advances in foundation modeling have reshaped computational pathology. However, the increasing number of available models and lack of standardized benchmarks make it increasingly complex to assess their strengths, limitations, and potential for further development. To address these challenges, we introduce a new suite of software tools for whole-slide image processing, foundation model benchmarking, and curated publicly available tasks. We anticipate that these resources will promote transparency, reproducibility, and continued progress in the field.
Molecular-driven Foundation Model for Oncologic Pathology
Jan 28, 2025Abstract:Foundation models are reshaping computational pathology by enabling transfer learning, where models pre-trained on vast datasets can be adapted for downstream diagnostic, prognostic, and therapeutic response tasks. Despite these advances, foundation models are still limited in their ability to encode the entire gigapixel whole-slide images without additional training and often lack complementary multimodal data. Here, we introduce Threads, a slide-level foundation model capable of generating universal representations of whole-slide images of any size. Threads was pre-trained using a multimodal learning approach on a diverse cohort of 47,171 hematoxylin and eosin (H&E)-stained tissue sections, paired with corresponding genomic and transcriptomic profiles - the largest such paired dataset to be used for foundation model development to date. This unique training paradigm enables Threads to capture the tissue's underlying molecular composition, yielding powerful representations applicable to a wide array of downstream tasks. In extensive benchmarking across 54 oncology tasks, including clinical subtyping, grading, mutation prediction, immunohistochemistry status determination, treatment response prediction, and survival prediction, Threads outperformed all baselines while demonstrating remarkable generalizability and label efficiency. It is particularly well suited for predicting rare events, further emphasizing its clinical utility. We intend to make the model publicly available for the broader community.
Multimodal Whole Slide Foundation Model for Pathology
Nov 29, 2024



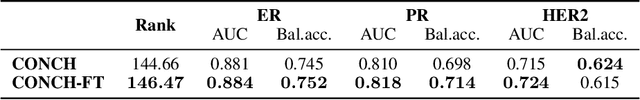
Abstract:The field of computational pathology has been transformed with recent advances in foundation models that encode histopathology region-of-interests (ROIs) into versatile and transferable feature representations via self-supervised learning (SSL). However, translating these advancements to address complex clinical challenges at the patient and slide level remains constrained by limited clinical data in disease-specific cohorts, especially for rare clinical conditions. We propose TITAN, a multimodal whole slide foundation model pretrained using 335,645 WSIs via visual self-supervised learning and vision-language alignment with corresponding pathology reports and 423,122 synthetic captions generated from a multimodal generative AI copilot for pathology. Without any finetuning or requiring clinical labels, TITAN can extract general-purpose slide representations and generate pathology reports that generalize to resource-limited clinical scenarios such as rare disease retrieval and cancer prognosis. We evaluate TITAN on diverse clinical tasks and find that TITAN outperforms both ROI and slide foundation models across machine learning settings such as linear probing, few-shot and zero-shot classification, rare cancer retrieval and cross-modal retrieval, and pathology report generation.
Multistain Pretraining for Slide Representation Learning in Pathology
Aug 05, 2024Abstract:Developing self-supervised learning (SSL) models that can learn universal and transferable representations of H&E gigapixel whole-slide images (WSIs) is becoming increasingly valuable in computational pathology. These models hold the potential to advance critical tasks such as few-shot classification, slide retrieval, and patient stratification. Existing approaches for slide representation learning extend the principles of SSL from small images (e.g., 224 x 224 patches) to entire slides, usually by aligning two different augmentations (or views) of the slide. Yet the resulting representation remains constrained by the limited clinical and biological diversity of the views. Instead, we postulate that slides stained with multiple markers, such as immunohistochemistry, can be used as different views to form a rich task-agnostic training signal. To this end, we introduce Madeleine, a multimodal pretraining strategy for slide representation learning. Madeleine is trained with a dual global-local cross-stain alignment objective on large cohorts of breast cancer samples (N=4,211 WSIs across five stains) and kidney transplant samples (N=12,070 WSIs across four stains). We demonstrate the quality of slide representations learned by Madeleine on various downstream evaluations, ranging from morphological and molecular classification to prognostic prediction, comprising 21 tasks using 7,299 WSIs from multiple medical centers. Code is available at https://github.com/mahmoodlab/MADELEINE.
Multimodal Prototyping for cancer survival prediction
Jun 28, 2024



Abstract:Multimodal survival methods combining gigapixel histology whole-slide images (WSIs) and transcriptomic profiles are particularly promising for patient prognostication and stratification. Current approaches involve tokenizing the WSIs into smaller patches (>10,000 patches) and transcriptomics into gene groups, which are then integrated using a Transformer for predicting outcomes. However, this process generates many tokens, which leads to high memory requirements for computing attention and complicates post-hoc interpretability analyses. Instead, we hypothesize that we can: (1) effectively summarize the morphological content of a WSI by condensing its constituting tokens using morphological prototypes, achieving more than 300x compression; and (2) accurately characterize cellular functions by encoding the transcriptomic profile with biological pathway prototypes, all in an unsupervised fashion. The resulting multimodal tokens are then processed by a fusion network, either with a Transformer or an optimal transport cross-alignment, which now operates with a small and fixed number of tokens without approximations. Extensive evaluation on six cancer types shows that our framework outperforms state-of-the-art methods with much less computation while unlocking new interpretability analyses.
HEST-1k: A Dataset for Spatial Transcriptomics and Histology Image Analysis
Jun 23, 2024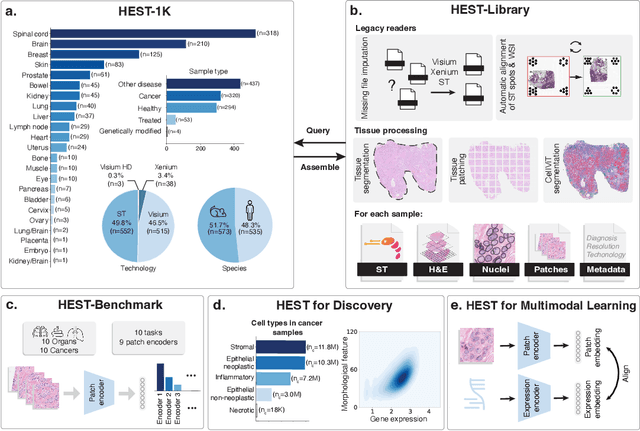
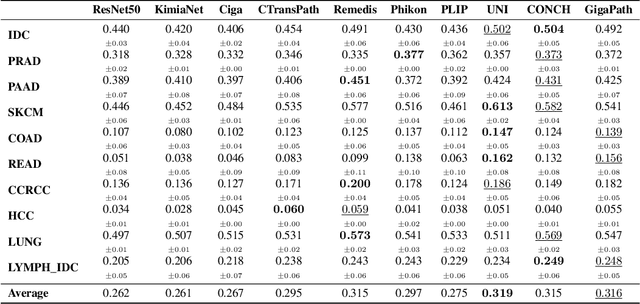
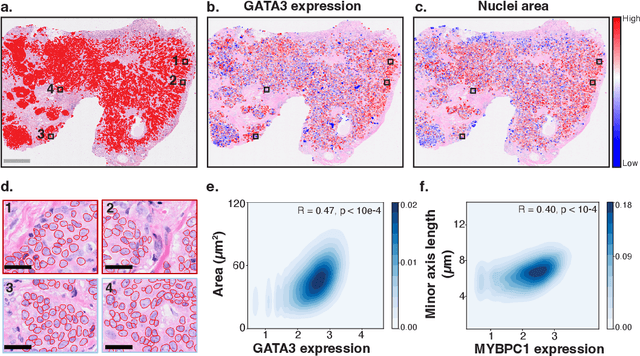
Abstract:Spatial transcriptomics (ST) enables interrogating the molecular composition of tissue with ever-increasing resolution, depth, and sensitivity. However, costs, rapidly evolving technology, and lack of standards have constrained computational methods in ST to narrow tasks and small cohorts. In addition, the underlying tissue morphology as reflected by H&E-stained whole slide images (WSIs) encodes rich information often overlooked in ST studies. Here, we introduce HEST-1k, a collection of 1,108 spatial transcriptomic profiles, each linked to a WSI and metadata. HEST-1k was assembled using HEST-Library from 131 public and internal cohorts encompassing 25 organs, two species (Homo Sapiens and Mus Musculus), and 320 cancer samples from 25 cancer types. HEST-1k processing enabled the identification of 1.5 million expression--morphology pairs and 60 million nuclei. HEST-1k is tested on three use cases: (1) benchmarking foundation models for histopathology (HEST-Benchmark), (2) biomarker identification, and (3) multimodal representation learning. HEST-1k, HEST-Library, and HEST-Benchmark can be freely accessed via https://github.com/mahmoodlab/hest.
Morphological Prototyping for Unsupervised Slide Representation Learning in Computational Pathology
May 19, 2024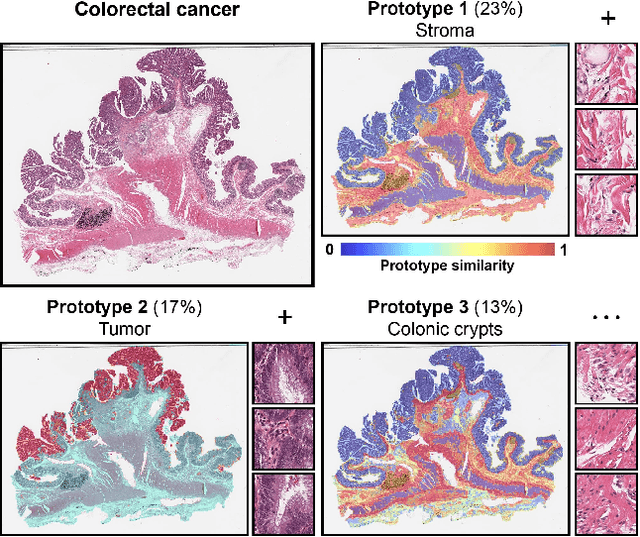
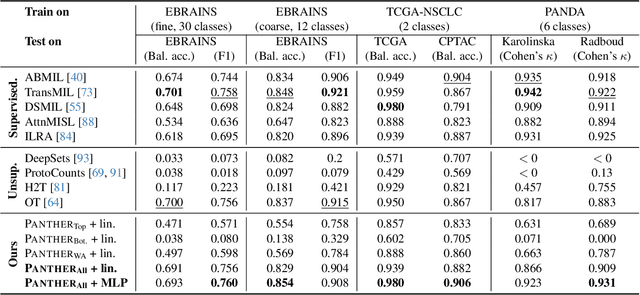
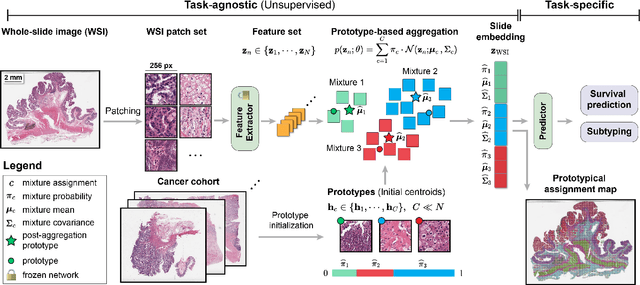
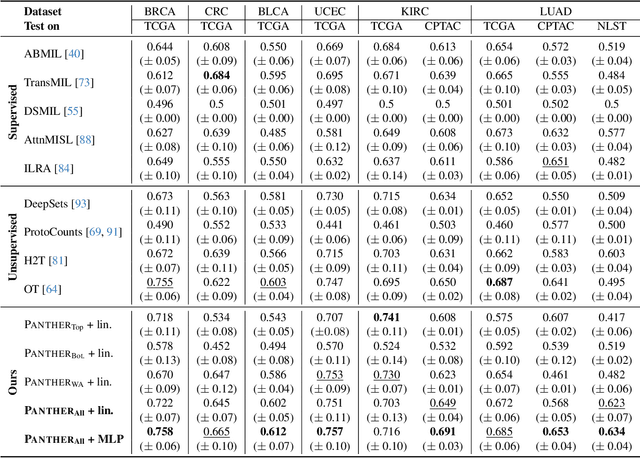
Abstract:Representation learning of pathology whole-slide images (WSIs) has been has primarily relied on weak supervision with Multiple Instance Learning (MIL). However, the slide representations resulting from this approach are highly tailored to specific clinical tasks, which limits their expressivity and generalization, particularly in scenarios with limited data. Instead, we hypothesize that morphological redundancy in tissue can be leveraged to build a task-agnostic slide representation in an unsupervised fashion. To this end, we introduce PANTHER, a prototype-based approach rooted in the Gaussian mixture model that summarizes the set of WSI patches into a much smaller set of morphological prototypes. Specifically, each patch is assumed to have been generated from a mixture distribution, where each mixture component represents a morphological exemplar. Utilizing the estimated mixture parameters, we then construct a compact slide representation that can be readily used for a wide range of downstream tasks. By performing an extensive evaluation of PANTHER on subtyping and survival tasks using 13 datasets, we show that 1) PANTHER outperforms or is on par with supervised MIL baselines and 2) the analysis of morphological prototypes brings new qualitative and quantitative insights into model interpretability.
Transcriptomics-guided Slide Representation Learning in Computational Pathology
May 19, 2024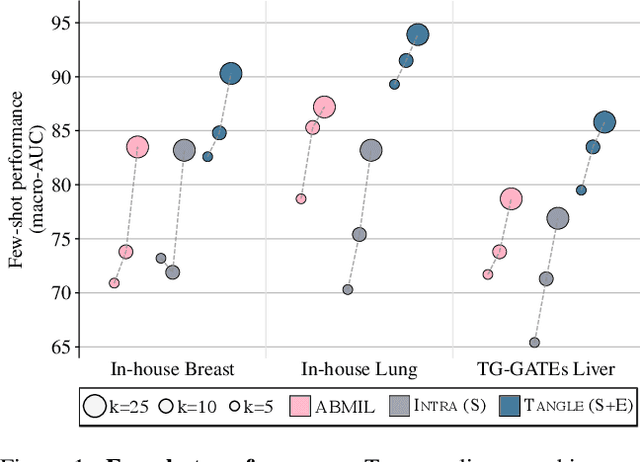
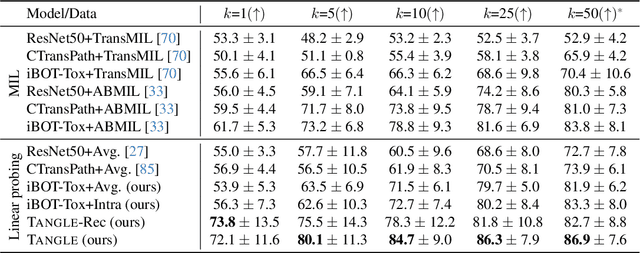
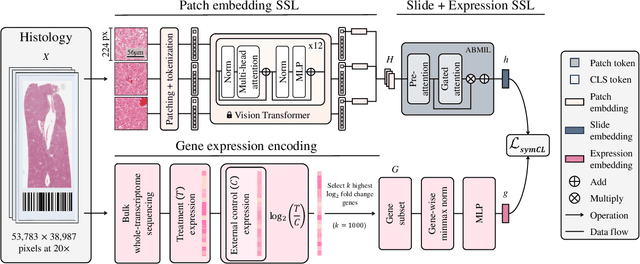
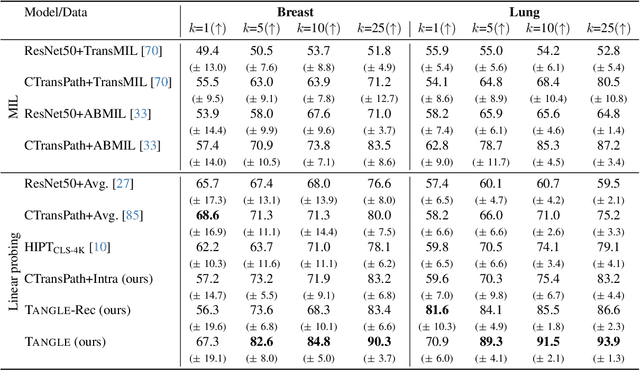
Abstract:Self-supervised learning (SSL) has been successful in building patch embeddings of small histology images (e.g., 224x224 pixels), but scaling these models to learn slide embeddings from the entirety of giga-pixel whole-slide images (WSIs) remains challenging. Here, we leverage complementary information from gene expression profiles to guide slide representation learning using multimodal pre-training. Expression profiles constitute highly detailed molecular descriptions of a tissue that we hypothesize offer a strong task-agnostic training signal for learning slide embeddings. Our slide and expression (S+E) pre-training strategy, called Tangle, employs modality-specific encoders, the outputs of which are aligned via contrastive learning. Tangle was pre-trained on samples from three different organs: liver (n=6,597 S+E pairs), breast (n=1,020), and lung (n=1,012) from two different species (Homo sapiens and Rattus norvegicus). Across three independent test datasets consisting of 1,265 breast WSIs, 1,946 lung WSIs, and 4,584 liver WSIs, Tangle shows significantly better few-shot performance compared to supervised and SSL baselines. When assessed using prototype-based classification and slide retrieval, Tangle also shows a substantial performance improvement over all baselines. Code available at https://github.com/mahmoodlab/TANGLE.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge