Yuchao Zheng
HyFormer: Revisiting the Roles of Sequence Modeling and Feature Interaction in CTR Prediction
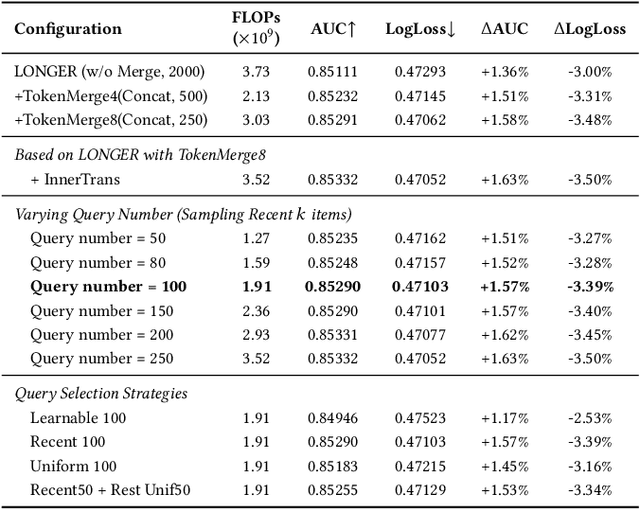
Jan 19, 2026Abstract:Industrial large-scale recommendation models (LRMs) face the challenge of jointly modeling long-range user behavior sequences and heterogeneous non-sequential features under strict efficiency constraints. However, most existing architectures employ a decoupled pipeline: long sequences are first compressed with a query-token based sequence compressor like LONGER, followed by fusion with dense features through token-mixing modules like RankMixer, which thereby limits both the representation capacity and the interaction flexibility. This paper presents HyFormer, a unified hybrid transformer architecture that tightly integrates long-sequence modeling and feature interaction into a single backbone. From the perspective of sequence modeling, we revisit and redesign query tokens in LRMs, and frame the LRM modeling task as an alternating optimization process that integrates two core components: Query Decoding which expands non-sequential features into Global Tokens and performs long sequence decoding over layer-wise key-value representations of long behavioral sequences; and Query Boosting which enhances cross-query and cross-sequence heterogeneous interactions via efficient token mixing. The two complementary mechanisms are performed iteratively to refine semantic representations across layers. Extensive experiments on billion-scale industrial datasets demonstrate that HyFormer consistently outperforms strong LONGER and RankMixer baselines under comparable parameter and FLOPs budgets, while exhibiting superior scaling behavior with increasing parameters and FLOPs. Large-scale online A/B tests in high-traffic production systems further validate its effectiveness, showing significant gains over deployed state-of-the-art models. These results highlight the practicality and scalability of HyFormer as a unified modeling framework for industrial LRMs.
LEMUR: Large scale End-to-end MUltimodal Recommendation
Nov 17, 2025Abstract:Traditional ID-based recommender systems often struggle with cold-start and generalization challenges. Multimodal recommendation systems, which leverage textual and visual data, offer a promising solution to mitigate these issues. However, existing industrial approaches typically adopt a two-stage training paradigm: first pretraining a multimodal model, then applying its frozen representations to train the recommendation model. This decoupled framework suffers from misalignment between multimodal learning and recommendation objectives, as well as an inability to adapt dynamically to new data. To address these limitations, we propose LEMUR, the first large-scale multimodal recommender system trained end-to-end from raw data. By jointly optimizing both the multimodal and recommendation components, LEMUR ensures tighter alignment with downstream objectives while enabling real-time parameter updates. Constructing multimodal sequential representations from user history often entails prohibitively high computational costs. To alleviate this bottleneck, we propose a novel memory bank mechanism that incrementally accumulates historical multimodal representations throughout the training process. After one month of deployment in Douyin Search, LEMUR has led to a 0.843% reduction in query change rate decay and a 0.81% improvement in QAUC. Additionally, LEMUR has shown significant gains across key offline metrics for Douyin Advertisement. Our results validate the superiority of end-to-end multimodal recommendation in real-world industrial scenarios.
SurGSplat: Progressive Geometry-Constrained Gaussian Splatting for Surgical Scene Reconstruction
Jun 06, 2025Abstract:Intraoperative navigation relies heavily on precise 3D reconstruction to ensure accuracy and safety during surgical procedures. However, endoscopic scenarios present unique challenges, including sparse features and inconsistent lighting, which render many existing Structure-from-Motion (SfM)-based methods inadequate and prone to reconstruction failure. To mitigate these constraints, we propose SurGSplat, a novel paradigm designed to progressively refine 3D Gaussian Splatting (3DGS) through the integration of geometric constraints. By enabling the detailed reconstruction of vascular structures and other critical features, SurGSplat provides surgeons with enhanced visual clarity, facilitating precise intraoperative decision-making. Experimental evaluations demonstrate that SurGSplat achieves superior performance in both novel view synthesis (NVS) and pose estimation accuracy, establishing it as a high-fidelity and efficient solution for surgical scene reconstruction. More information and results can be found on the page https://surgsplat.github.io/.
LONGER: Scaling Up Long Sequence Modeling in Industrial Recommenders
May 07, 2025

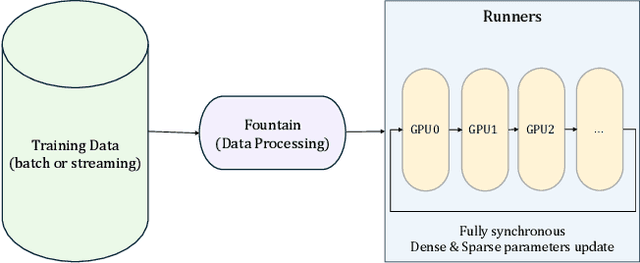
Abstract:Modeling ultra-long user behavior sequences is critical for capturing both long- and short-term preferences in industrial recommender systems. Existing solutions typically rely on two-stage retrieval or indirect modeling paradigms, incuring upstream-downstream inconsistency and computational inefficiency. In this paper, we present LONGER, a Long-sequence Optimized traNsformer for GPU-Efficient Recommenders. LONGER incorporates (i) a global token mechanism for stabilizing attention over long contexts, (ii) a token merge module with lightweight InnerTransformers and hybrid attention strategy to reduce quadratic complexity, and (iii) a series of engineering optimizations, including training with mixed-precision and activation recomputation, KV cache serving, and the fully synchronous model training and serving framework for unified GPU-based dense and sparse parameter updates. LONGER consistently outperforms strong baselines in both offline metrics and online A/B testing in both advertising and e-commerce services at ByteDance, validating its consistent effectiveness and industrial-level scaling laws. Currently, LONGER has been fully deployed at more than 10 influential scenarios at ByteDance, serving billion users.
Large Memory Network for Recommendation
Feb 08, 2025



Abstract:Modeling user behavior sequences in recommender systems is essential for understanding user preferences over time, enabling personalized and accurate recommendations for improving user retention and enhancing business values. Despite its significance, there are two challenges for current sequential modeling approaches. From the spatial dimension, it is difficult to mutually perceive similar users' interests for a generalized intention understanding; from the temporal dimension, current methods are generally prone to forgetting long-term interests due to the fixed-length input sequence. In this paper, we present Large Memory Network (LMN), providing a novel idea by compressing and storing user history behavior information in a large-scale memory block. With the elaborated online deployment strategy, the memory block can be easily scaled up to million-scale in the industry. Extensive offline comparison experiments, memory scaling up experiments, and online A/B test on Douyin E-Commerce Search (ECS) are performed, validating the superior performance of LMN. Currently, LMN has been fully deployed in Douyin ECS, serving millions of users each day.
GBR: Generative Bundle Refinement for High-fidelity Gaussian Splatting and Meshing
Dec 08, 2024



Abstract:Gaussian splatting has gained attention for its efficient representation and rendering of 3D scenes using continuous Gaussian primitives. However, it struggles with sparse-view inputs due to limited geometric and photometric information, causing ambiguities in depth, shape, and texture. we propose GBR: Generative Bundle Refinement, a method for high-fidelity Gaussian splatting and meshing using only 4-6 input views. GBR integrates a neural bundle adjustment module to enhance geometry accuracy and a generative depth refinement module to improve geometry fidelity. More specifically, the neural bundle adjustment module integrates a foundation network to produce initial 3D point maps and point matches from unposed images, followed by bundle adjustment optimization to improve multiview consistency and point cloud accuracy. The generative depth refinement module employs a diffusion-based strategy to enhance geometric details and fidelity while preserving the scale. Finally, for Gaussian splatting optimization, we propose a multimodal loss function incorporating depth and normal consistency, geometric regularization, and pseudo-view supervision, providing robust guidance under sparse-view conditions. Experiments on widely used datasets show that GBR significantly outperforms existing methods under sparse-view inputs. Additionally, GBR demonstrates the ability to reconstruct and render large-scale real-world scenes, such as the Pavilion of Prince Teng and the Great Wall, with remarkable details using only 6 views.
Multimodal 3D Brain Tumor Segmentation with Adversarial Training and Conditional Random Field
Nov 21, 2024Abstract:Accurate brain tumor segmentation remains a challenging task due to structural complexity and great individual differences of gliomas. Leveraging the pre-eminent detail resilience of CRF and spatial feature extraction capacity of V-net, we propose a multimodal 3D Volume Generative Adversarial Network (3D-vGAN) for precise segmentation. The model utilizes Pseudo-3D for V-net improvement, adds conditional random field after generator and use original image as supplemental guidance. Results, using the BraTS-2018 dataset, show that 3D-vGAN outperforms classical segmentation models, including U-net, Gan, FCN and 3D V-net, reaching specificity over 99.8%.
* 13 pages, 7 figures, Annual Conference on Medical Image Understanding and Analysis (MIUA) 2024
Application of Graph Based Features in Computer Aided Diagnosis for Histopathological Image Classification of Gastric Cancer
May 17, 2022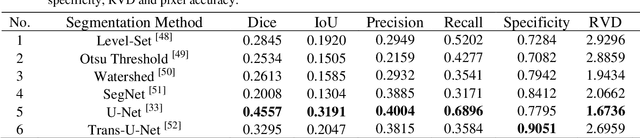
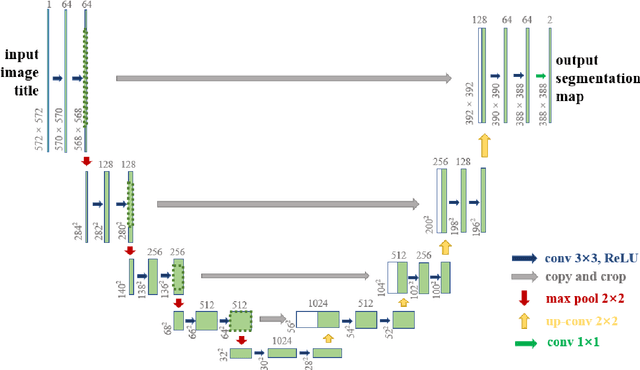

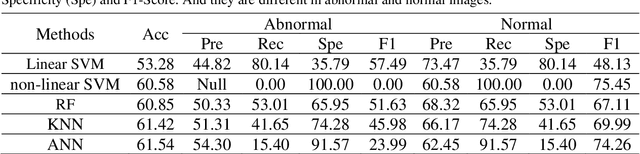
Abstract:The gold standard for gastric cancer detection is gastric histopathological image analysis, but there are certain drawbacks in the existing histopathological detection and diagnosis. In this paper, based on the study of computer aided diagnosis system, graph based features are applied to gastric cancer histopathology microscopic image analysis, and a classifier is used to classify gastric cancer cells from benign cells. Firstly, image segmentation is performed, and after finding the region, cell nuclei are extracted using the k-means method, the minimum spanning tree (MST) is drawn, and graph based features of the MST are extracted. The graph based features are then put into the classifier for classification. In this study, different segmentation methods are compared in the tissue segmentation stage, among which are Level-Set, Otsu thresholding, watershed, SegNet, U-Net and Trans-U-Net segmentation; Graph based features, Red, Green, Blue features, Grey-Level Co-occurrence Matrix features, Histograms of Oriented Gradient features and Local Binary Patterns features are compared in the feature extraction stage; Radial Basis Function (RBF) Support Vector Machine (SVM), Linear SVM, Artificial Neural Network, Random Forests, k-NearestNeighbor, VGG16, and Inception-V3 are compared in the classifier stage. It is found that using U-Net to segment tissue areas, then extracting graph based features, and finally using RBF SVM classifier gives the optimal results with 94.29%.
Application of Transfer Learning and Ensemble Learning in Image-level Classification for Breast Histopathology
Apr 18, 2022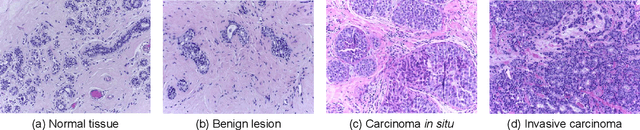

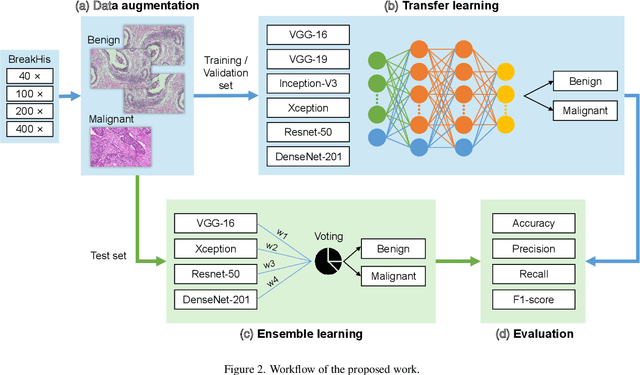
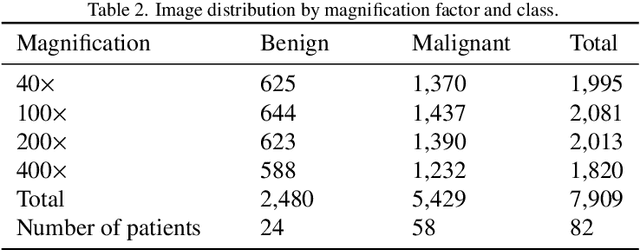
Abstract:Background: Breast cancer has the highest prevalence in women globally. The classification and diagnosis of breast cancer and its histopathological images have always been a hot spot of clinical concern. In Computer-Aided Diagnosis (CAD), traditional classification models mostly use a single network to extract features, which has significant limitations. On the other hand, many networks are trained and optimized on patient-level datasets, ignoring the application of lower-level data labels. Method: This paper proposes a deep ensemble model based on image-level labels for the binary classification of benign and malignant lesions of breast histopathological images. First, the BreakHis dataset is randomly divided into a training, validation and test set. Then, data augmentation techniques are used to balance the number of benign and malignant samples. Thirdly, considering the performance of transfer learning and the complementarity between each network, VGG-16, Xception, Resnet-50, DenseNet-201 are selected as the base classifiers. Result: In the ensemble network model with accuracy as the weight, the image-level binary classification achieves an accuracy of $98.90\%$. In order to verify the capabilities of our method, the latest Transformer and Multilayer Perception (MLP) models have been experimentally compared on the same dataset. Our model wins with a $5\%-20\%$ advantage, emphasizing the ensemble model's far-reaching significance in classification tasks. Conclusion: This research focuses on improving the model's classification performance with an ensemble algorithm. Transfer learning plays an essential role in small datasets, improving training speed and accuracy. Our model has outperformed many existing approaches in accuracy, providing a method for the field of auxiliary medical diagnosis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge