Shijia Geng
ECGomics: An Open Platform for AI-ECG Digital Biomarker Discovery
Jan 19, 2026Abstract:Background: Conventional electrocardiogram (ECG) analysis faces a persistent dichotomy: expert-driven features ensure interpretability but lack sensitivity to latent patterns, while deep learning offers high accuracy but functions as a black box with high data dependency. We introduce ECGomics, a systematic paradigm and open-source platform for the multidimensional deconstruction of cardiac signals into digital biomarker. Methods: Inspired by the taxonomic rigor of genomics, ECGomics deconstructs cardiac activity across four dimensions: Structural, Intensity, Functional, and Comparative. This taxonomy synergizes expert-defined morphological rules with data-driven latent representations, effectively bridging the gap between handcrafted features and deep learning embeddings. Results: We operationalized this framework into a scalable ecosystem consisting of a web-based research platform and a mobile-integrated solution (https://github.com/PKUDigitalHealth/ECGomics). The web platform facilitates high-throughput analysis via precision parameter configuration, high-fidelity data ingestion, and 12-lead visualization, allowing for the systematic extraction of biomarkers across the four ECGomics dimensions. Complementarily, the mobile interface, integrated with portable sensors and a cloud-based engine, enables real-time signal acquisition and near-instantaneous delivery of structured diagnostic reports. This dual-interface architecture successfully transitions ECGomics from theoretical discovery to decentralized, real-world health management, ensuring professional-grade monitoring in diverse clinical and home-based settings. Conclusion: ECGomics harmonizes diagnostic precision, interpretability, and data efficiency. By providing a deployable software ecosystem, this paradigm establishes a robust foundation for digital biomarker discovery and personalized cardiovascular medicine.
Artificial Intelligence-Enabled Spirometry for Early Detection of Right Heart Failure
Nov 17, 2025Abstract:Right heart failure (RHF) is a disease characterized by abnormalities in the structure or function of the right ventricle (RV), which is associated with high morbidity and mortality. Lung disease often causes increased right ventricular load, leading to RHF. Therefore, it is very important to screen out patients with cor pulmonale who develop RHF from people with underlying lung diseases. In this work, we propose a self-supervised representation learning method to early detecting RHF from patients with cor pulmonale, which uses spirogram time series to predict patients with RHF at an early stage. The proposed model is divided into two stages. The first stage is the self-supervised representation learning-based spirogram embedding (SLSE) network training process, where the encoder of the Variational autoencoder (VAE-encoder) learns a robust low-dimensional representation of the spirogram time series from the data-augmented unlabeled data. Second, this low-dimensional representation is fused with demographic information and fed into a CatBoost classifier for the downstream RHF prediction task. Trained and tested on a carefully selected subset of 26,617 individuals from the UK Biobank, our model achieved an AUROC of 0.7501 in detecting RHF, demonstrating strong population-level distinction ability. We further evaluated the model on high-risk clinical subgroups, achieving AUROC values of 0.8194 on a test set of 74 patients with chronic kidney disease (CKD) and 0.8413 on a set of 64 patients with valvular heart disease (VHD). These results highlight the model's potential utility in predicting RHF among clinically elevated-risk populations. In conclusion, this study presents a self-supervised representation learning approach combining spirogram time series and demographic data, demonstrating promising potential for early RHF detection in clinical practice.
Accuracy of Wearable ECG Parameter Calculation Method for Long QT and First-Degree A-V Block Detection: A Multi-Center Real-World Study with External Validations Compared to Standard ECG Machines and Cardiologist Assessments
Feb 21, 2025Abstract:In recent years, wearable devices have revolutionized cardiac monitoring by enabling continuous, non-invasive ECG recording in real-world settings. Despite these advances, the accuracy of ECG parameter calculations (PR interval, QRS interval, QT interval, etc.) from wearables remains to be rigorously validated against conventional ECG machines and expert clinician assessments. In this large-scale, multicenter study, we evaluated FeatureDB, a novel algorithm for automated computation of ECG parameters from wearable single-lead signals Three diverse datasets were employed: the AHMU-FH dataset (n=88,874), the CSE dataset (n=106), and the HeartVoice-ECG-lite dataset (n=369) with annotations provided by two experienced cardiologists. FeatureDB demonstrates a statistically significant correlation with key parameters (PR interval, QRS duration, QT interval, and QTc) calculated by standard ECG machines and annotated by clinical doctors. Bland-Altman analysis confirms a high level of agreement.Moreover,FeatureDB exhibited robust diagnostic performance in detecting Long QT syndrome (LQT) and atrioventricular block interval abnormalities (AVBI),with excellent area under the ROC curve (LQT: 0.836, AVBI: 0.861),accuracy (LQT: 0.856, AVBI: 0.845),sensitivity (LQT: 0.815, AVBI: 0.877),and specificity (LQT: 0.856, AVBI: 0.845).This further validates its clinical reliability. These results validate the clinical applicability of FeatureDB for wearable ECG analysis and highlight its potential to bridge the gap between traditional diagnostic methods and emerging wearable technologies.Ultimately,this study supports integrating wearable ECG devices into large-scale cardiovascular disease management and early intervention strategies,and it highlights the potential of wearable ECG technologies to deliver accurate,clinically relevant cardiac monitoring while advancing broader applications in cardiovascular care.
DiffuSETS: 12-lead ECG Generation Conditioned on Clinical Text Reports and Patient-Specific Information
Jan 10, 2025Abstract:Heart disease remains a significant threat to human health. As a non-invasive diagnostic tool, the electrocardiogram (ECG) is one of the most widely used methods for cardiac screening. However, the scarcity of high-quality ECG data, driven by privacy concerns and limited medical resources, creates a pressing need for effective ECG signal generation. Existing approaches for generating ECG signals typically rely on small training datasets, lack comprehensive evaluation frameworks, and overlook potential applications beyond data augmentation. To address these challenges, we propose DiffuSETS, a novel framework capable of generating ECG signals with high semantic alignment and fidelity. DiffuSETS accepts various modalities of clinical text reports and patient-specific information as inputs, enabling the creation of clinically meaningful ECG signals. Additionally, to address the lack of standardized evaluation in ECG generation, we introduce a comprehensive benchmarking methodology to assess the effectiveness of generative models in this domain. Our model achieve excellent results in tests, proving its superiority in the task of ECG generation. Furthermore, we showcase its potential to mitigate data scarcity while exploring novel applications in cardiology education and medical knowledge discovery, highlighting the broader impact of our work.
Deep Learning for Detecting and Early Predicting Chronic Obstructive Pulmonary Disease from Spirogram Time Series: A UK Biobank Study
May 06, 2024



Abstract:Chronic Obstructive Pulmonary Disease (COPD) is a chronic inflammatory lung condition that causes airflow obstruction. The existing methods can only detect patients who already have COPD based on obvious features shown in the spirogram (In this article, the spirogram specifically involves measuring Volume-Flow curve time series). Early prediction of COPD risk is vital for monitoring COPD disease progression, slowing it down, or even preventing its onset. However, these methods fail to early predict an individual's probability of COPD in the future based on subtle features in the spirogram. To address this gap, for the first time, we propose DeepSpiro, a method based on deep learning for early prediction of future COPD risk. DeepSpiro consists of four parts. First, we construct Volume-Flow curves guided by Time-Volume instability smoothing (SpiroSmoother) to enhance the stability of the original Volume-Flow curves precisely. Second, we extract critical features from the evolution of varied-length key patches (SpiroEncoder) to capture the key temporal evolution from original high-dimensional dynamic sequences to a unified low-dimensional temporal representation. Third, we explain the model based on temporal attention and heterogeneous feature fusion (SpiroExplainer), which integrates information from heterogeneous data such as spirogram and demographic information. Fourth, we predict the risk of COPD based on the evolution of key patch concavity (SpiroPredictor), enabling accurate prediction of the risk of disease in high-risk patients who are not yet diagnosed, for up to 1, 2, 3, 4, 5 years, and beyond. We conduct experiments on the UK Biobank dataset. Results show that DeepSpiro achieves an AUC value of 0.8328 in the task of detecting COPD. In early prediction tasks, high-risk and low-risk groups show significant differences in the future, with a p-value of <0.001.
A Deep Learning Method for Beat-Level Risk Analysis and Interpretation of Atrial Fibrillation Patients during Sinus Rhythm
Mar 18, 2024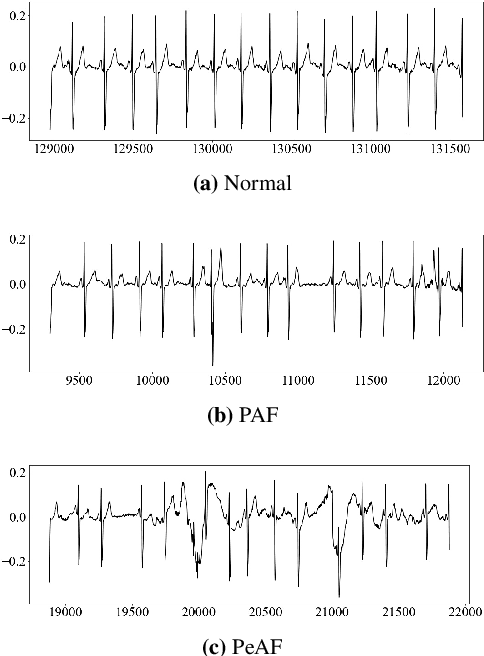

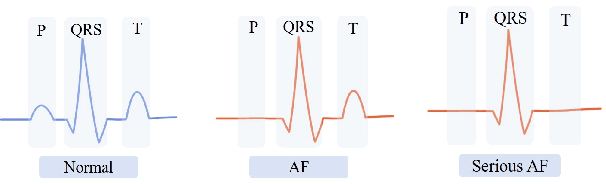
Abstract:Atrial Fibrillation (AF) is a common cardiac arrhythmia. Many AF patients experience complications such as stroke and other cardiovascular issues. Early detection of AF is crucial. Existing algorithms can only distinguish ``AF rhythm in AF patients'' from ``sinus rhythm in normal individuals'' . However, AF patients do not always exhibit AF rhythm, posing a challenge for diagnosis when the AF rhythm is absent. To address this, this paper proposes a novel artificial intelligence (AI) algorithm to distinguish ``sinus rhythm in AF patients'' and ``sinus rhythm in normal individuals'' in beat-level. We introduce beat-level risk interpreters, trend risk interpreters, addressing the interpretability issues of deep learning models and the difficulty in explaining AF risk trends. Additionally, the beat-level information fusion decision is presented to enhance model accuracy. The experimental results demonstrate that the average AUC for single beats used as testing data from CPSC 2021 dataset is 0.7314. By employing 150 beats for information fusion decision algorithm, the average AUC can reach 0.7591. Compared to previous segment-level algorithms, we utilized beats as input, reducing data dimensionality and making the model more lightweight, facilitating deployment on portable medical devices. Furthermore, we draw new and interesting findings through average beat analysis and subgroup analysis, considering varying risk levels.
A Review of Deep Learning Methods for Photoplethysmography Data
Jan 23, 2024Abstract:Photoplethysmography (PPG) is a highly promising device due to its advantages in portability, user-friendly operation, and non-invasive capabilities to measure a wide range of physiological information. Recent advancements in deep learning have demonstrated remarkable outcomes by leveraging PPG signals for tasks related to personal health management and other multifaceted applications. In this review, we systematically reviewed papers that applied deep learning models to process PPG data between January 1st of 2017 and July 31st of 2023 from Google Scholar, PubMed and Dimensions. Each paper is analyzed from three key perspectives: tasks, models, and data. We finally extracted 193 papers where different deep learning frameworks were used to process PPG signals. Based on the tasks addressed in these papers, we categorized them into two major groups: medical-related, and non-medical-related. The medical-related tasks were further divided into seven subgroups, including blood pressure analysis, cardiovascular monitoring and diagnosis, sleep health, mental health, respiratory monitoring and analysis, blood glucose analysis, as well as others. The non-medical-related tasks were divided into four subgroups, which encompass signal processing, biometric identification, electrocardiogram reconstruction, and human activity recognition. In conclusion, significant progress has been made in the field of using deep learning methods to process PPG data recently. This allows for a more thorough exploration and utilization of the information contained in PPG signals. However, challenges remain, such as limited quantity and quality of publicly available databases, a lack of effective validation in real-world scenarios, and concerns about the interpretability, scalability, and complexity of deep learning models. Moreover, there are still emerging research areas that require further investigation.
Artificial Intelligence System for Detection and Screening of Cardiac Abnormalities using Electrocardiogram Images
Feb 10, 2023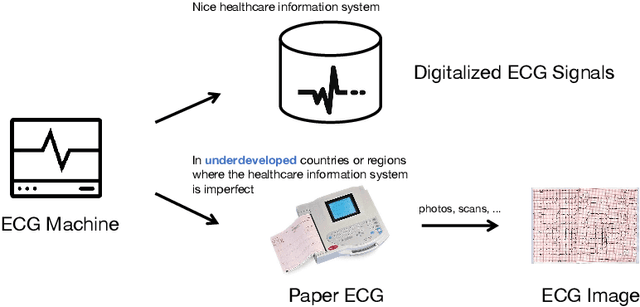



Abstract:The artificial intelligence (AI) system has achieved expert-level performance in electrocardiogram (ECG) signal analysis. However, in underdeveloped countries or regions where the healthcare information system is imperfect, only paper ECGs can be provided. Analysis of real-world ECG images (photos or scans of paper ECGs) remains challenging due to complex environments or interference. In this study, we present an AI system developed to detect and screen cardiac abnormalities (CAs) from real-world ECG images. The system was evaluated on a large dataset of 52,357 patients from multiple regions and populations across the world. On the detection task, the AI system obtained area under the receiver operating curve (AUC) of 0.996 (hold-out test), 0.994 (external test 1), 0.984 (external test 2), and 0.979 (external test 3), respectively. Meanwhile, the detection results of AI system showed a strong correlation with the diagnosis of cardiologists (cardiologist 1 (R=0.794, p<1e-3), cardiologist 2 (R=0.812, p<1e-3)). On the screening task, the AI system achieved AUCs of 0.894 (hold-out test) and 0.850 (external test). The screening performance of the AI system was better than that of the cardiologists (AI system (0.846) vs. cardiologist 1 (0.520) vs. cardiologist 2 (0.480)). Our study demonstrates the feasibility of an accurate, objective, easy-to-use, fast, and low-cost AI system for CA detection and screening. The system has the potential to be used by healthcare professionals, caregivers, and general users to assess CAs based on real-world ECG images.
Cross Reconstruction Transformer for Self-Supervised Time Series Representation Learning
May 20, 2022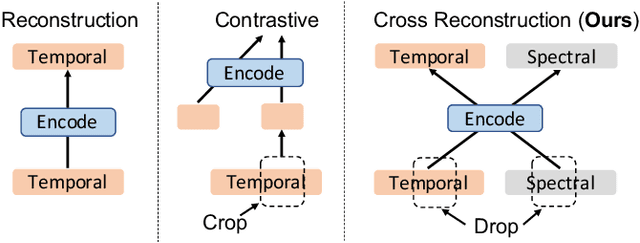
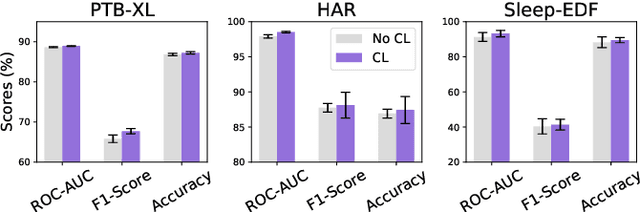

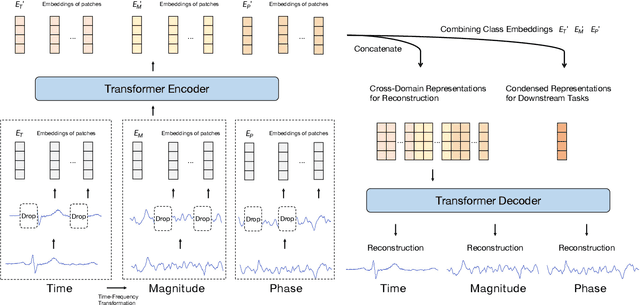
Abstract:Unsupervised/self-supervised representation learning in time series is critical since labeled samples are usually scarce in real-world scenarios. Existing approaches mainly leverage the contrastive learning framework, which automatically learns to understand the similar and dissimilar data pairs. Nevertheless, they are restricted to the prior knowledge of constructing pairs, cumbersome sampling policy, and unstable performances when encountering sampling bias. Also, few works have focused on effectively modeling across temporal-spectral relations to extend the capacity of representations. In this paper, we aim at learning representations for time series from a new perspective and propose Cross Reconstruction Transformer (CRT) to solve the aforementioned problems in a unified way. CRT achieves time series representation learning through a cross-domain dropping-reconstruction task. Specifically, we transform time series into the frequency domain and randomly drop certain parts in both time and frequency domains. Dropping can maximally preserve the global context compared to cropping and masking. Then a transformer architecture is utilized to adequately capture the cross-domain correlations between temporal and spectral information through reconstructing data in both domains, which is called Dropped Temporal-Spectral Modeling. To discriminate the representations in global latent space, we propose Instance Discrimination Constraint to reduce the mutual information between different time series and sharpen the decision boundaries. Additionally, we propose a specified curriculum learning strategy to optimize the CRT, which progressively increases the dropping ratio in the training process.
Defending Against Adversarial Attack in ECG Classification with Adversarial Distillation Training
Mar 14, 2022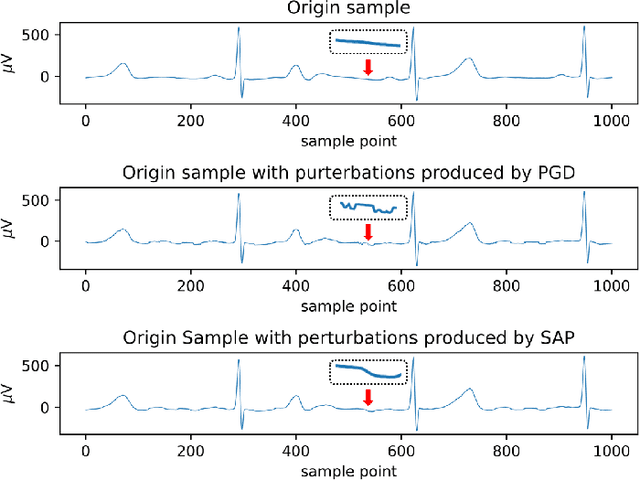
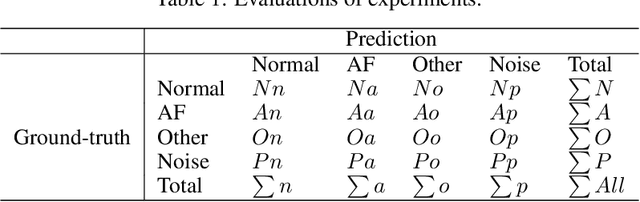
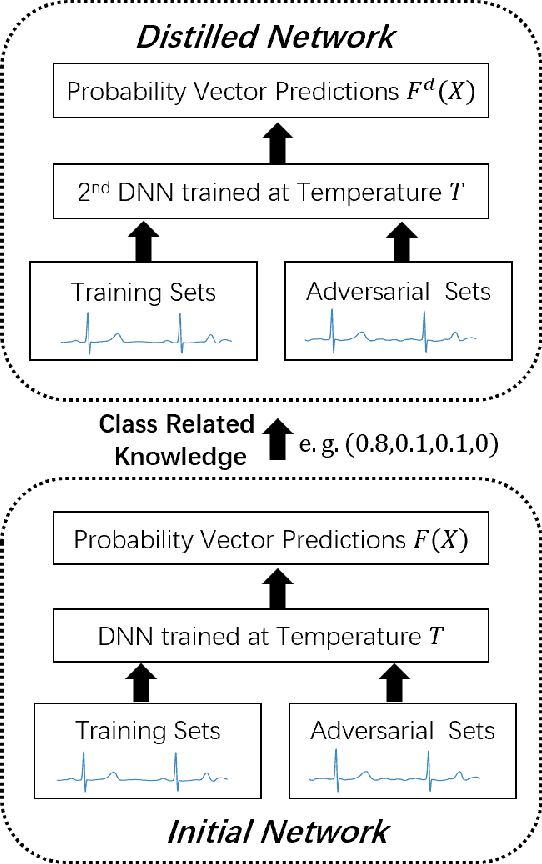
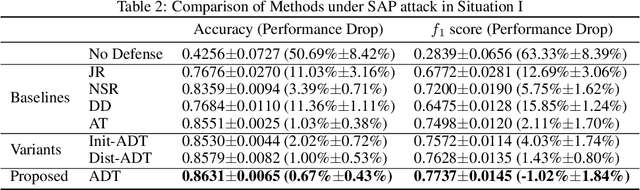
Abstract:In clinics, doctors rely on electrocardiograms (ECGs) to assess severe cardiac disorders. Owing to the development of technology and the increase in health awareness, ECG signals are currently obtained by using medical and commercial devices. Deep neural networks (DNNs) can be used to analyze these signals because of their high accuracy rate. However, researchers have found that adversarial attacks can significantly reduce the accuracy of DNNs. Studies have been conducted to defend ECG-based DNNs against traditional adversarial attacks, such as projected gradient descent (PGD), and smooth adversarial perturbation (SAP) which targets ECG classification; however, to the best of our knowledge, no study has completely explored the defense against adversarial attacks targeting ECG classification. Thus, we did different experiments to explore the effects of defense methods against white-box adversarial attack and black-box adversarial attack targeting ECG classification, and we found that some common defense methods performed well against these attacks. Besides, we proposed a new defense method called Adversarial Distillation Training (ADT) which comes from defensive distillation and can effectively improve the generalization performance of DNNs. The results show that our method performed more effectively against adversarial attacks targeting on ECG classification than the other baseline methods, namely, adversarial training, defensive distillation, Jacob regularization, and noise-to-signal ratio regularization. Furthermore, we found that our method performed better against PGD attacks with low noise levels, which means that our method has stronger robustness.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge