Ramandeep Singh
AdaptBot: Combining LLM with Knowledge Graphs and Human Input for Generic-to-Specific Task Decomposition and Knowledge Refinement
Feb 04, 2025Abstract:Embodied agents assisting humans are often asked to complete a new task in a new scenario. An agent preparing a particular dish in the kitchen based on a known recipe may be asked to prepare a new dish or to perform cleaning tasks in the storeroom. There may not be sufficient resources, e.g., time or labeled examples, to train the agent for these new situations. Large Language Models (LLMs) trained on considerable knowledge across many domains are able to predict a sequence of abstract actions for such new tasks and scenarios, although it may not be possible for the agent to execute this action sequence due to task-, agent-, or domain-specific constraints. Our framework addresses these challenges by leveraging the generic predictions provided by LLM and the prior domain-specific knowledge encoded in a Knowledge Graph (KG), enabling an agent to quickly adapt to new tasks and scenarios. The robot also solicits and uses human input as needed to refine its existing knowledge. Based on experimental evaluation over cooking and cleaning tasks in simulation domains, we demonstrate that the interplay between LLM, KG, and human input leads to substantial performance gains compared with just using the LLM output.
Anticipate & Collab: Data-driven Task Anticipation and Knowledge-driven Planning for Human-robot Collaboration
Apr 04, 2024



Abstract:An agent assisting humans in daily living activities can collaborate more effectively by anticipating upcoming tasks. Data-driven methods represent the state of the art in task anticipation, planning, and related problems, but these methods are resource-hungry and opaque. Our prior work introduced a proof of concept framework that used an LLM to anticipate 3 high-level tasks that served as goals for a classical planning system that computed a sequence of low-level actions for the agent to achieve these goals. This paper describes DaTAPlan, our framework that significantly extends our prior work toward human-robot collaboration. Specifically, DaTAPlan planner computes actions for an agent and a human to collaboratively and jointly achieve the tasks anticipated by the LLM, and the agent automatically adapts to unexpected changes in human action outcomes and preferences. We evaluate DaTAPlan capabilities in a realistic simulation environment, demonstrating accurate task anticipation, effective human-robot collaboration, and the ability to adapt to unexpected changes. Project website: https://dataplan-hrc.github.io
Deep Learning Predicts Cardiovascular Disease Risks from Lung Cancer Screening Low Dose Computed Tomography
Aug 16, 2020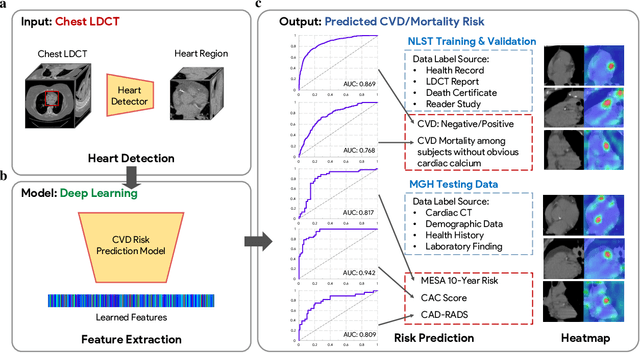

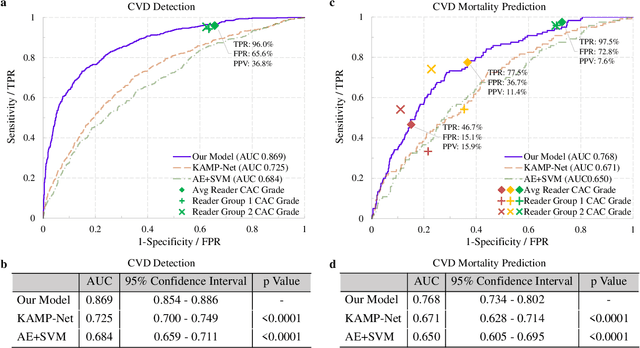

Abstract:The high risk population of cardiovascular disease (CVD) is simultaneously at high risk of lung cancer. Given the dominance of low dose computed tomography (LDCT) for lung cancer screening, the feasibility of extracting information on CVD from the same LDCT scan would add major value to patients at no additional radiation dose. However, with strong noise in LDCT images and without electrocardiogram (ECG) gating, CVD risk analysis from LDCT is highly challenging. Here we present an innovative deep learning model to address this challenge. Our deep model was trained with 30,286 LDCT volumes and achieved the state-of-the-art performance (area under the curve (AUC) of 0.869) on 2,085 National Lung Cancer Screening Trial (NLST) subjects, and effectively identified patients with high CVD mortality risks (AUC of 0.768). Our deep model was further calibrated against the clinical gold standard CVD risk scores from ECG-gated dedicated cardiac CT, including coronary artery calcification (CAC) score, CAD-RADS score and MESA 10-year CHD risk score from an independent dataset of 106 subjects. In this validation study, our model achieved AUC of 0.942, 0.809 and 0.817 for CAC, CAD-RADS and MESA scores, respectively. Our deep learning model has the potential to convert LDCT for lung cancer screening into dual-screening quantitative tool for CVD risk estimation.
Neuro-Endo-Trainer-Online Assessment System (NET-OAS) for Neuro-Endoscopic Skills Training
Jul 16, 2020



Abstract:Neuro-endoscopy is a challenging minimally invasive neurosurgery that requires surgical skills to be acquired using training methods different from the existing apprenticeship model. There are various training systems developed for imparting fundamental technical skills in laparoscopy where as limited systems for neuro-endoscopy. Neuro-Endo-Trainer was a box-trainer developed for endo-nasal transsphenoidal surgical skills training with video based offline evaluation system. The objective of the current study was to develop a modified version (Neuro-Endo-Trainer-Online Assessment System (NET-OAS)) by providing a stand-alone system with online evaluation and real-time feedback. The validation study on a group of 15 novice participants shows the improvement in the technical skills for handling the neuro-endoscope and the tool while performing pick and place activity.
* Published at Federated Conference on Computer Science and Information Systems - FedCSIS 2017
Quantifying and Leveraging Predictive Uncertainty for Medical Image Assessment
Jul 08, 2020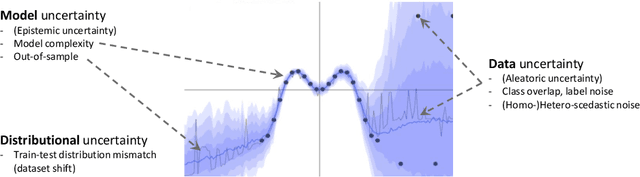
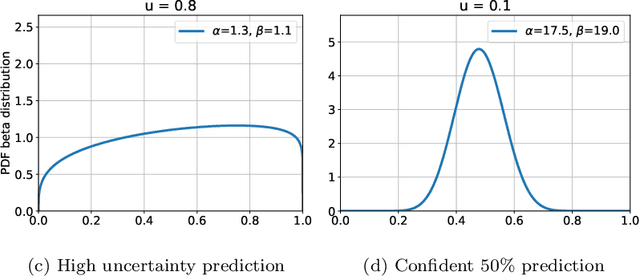
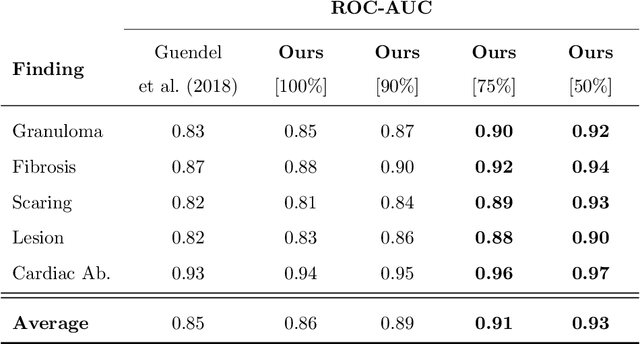
Abstract:The interpretation of medical images is a challenging task, often complicated by the presence of artifacts, occlusions, limited contrast and more. Most notable is the case of chest radiography, where there is a high inter-rater variability in the detection and classification of abnormalities. This is largely due to inconclusive evidence in the data or subjective definitions of disease appearance. An additional example is the classification of anatomical views based on 2D Ultrasound images. Often, the anatomical context captured in a frame is not sufficient to recognize the underlying anatomy. Current machine learning solutions for these problems are typically limited to providing probabilistic predictions, relying on the capacity of underlying models to adapt to limited information and the high degree of label noise. In practice, however, this leads to overconfident systems with poor generalization on unseen data. To account for this, we propose a system that learns not only the probabilistic estimate for classification, but also an explicit uncertainty measure which captures the confidence of the system in the predicted output. We argue that this approach is essential to account for the inherent ambiguity characteristic of medical images from different radiologic exams including computed radiography, ultrasonography and magnetic resonance imaging. In our experiments we demonstrate that sample rejection based on the predicted uncertainty can significantly improve the ROC-AUC for various tasks, e.g., by 8% to 0.91 with an expected rejection rate of under 25% for the classification of different abnormalities in chest radiographs. In addition, we show that using uncertainty-driven bootstrapping to filter the training data, one can achieve a significant increase in robustness and accuracy.
Quantifying and Leveraging Classification Uncertainty for Chest Radiograph Assessment
Jun 18, 2019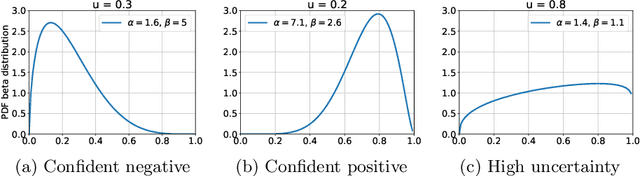
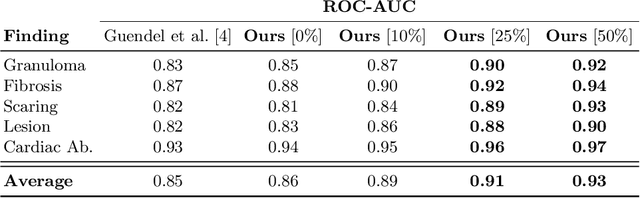
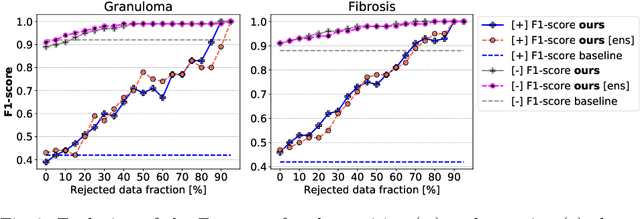
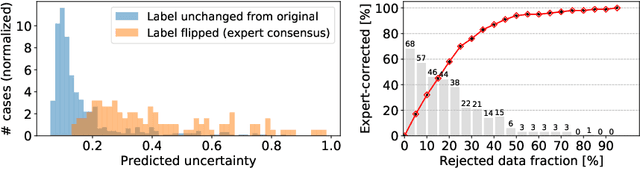
Abstract:The interpretation of chest radiographs is an essential task for the detection of thoracic diseases and abnormalities. However, it is a challenging problem with high inter-rater variability and inherent ambiguity due to inconclusive evidence in the data, limited data quality or subjective definitions of disease appearance. Current deep learning solutions for chest radiograph abnormality classification are typically limited to providing probabilistic predictions, relying on the capacity of learning models to adapt to the high degree of label noise and become robust to the enumerated causal factors. In practice, however, this leads to overconfident systems with poor generalization on unseen data. To account for this, we propose an automatic system that learns not only the probabilistic estimate on the presence of an abnormality, but also an explicit uncertainty measure which captures the confidence of the system in the predicted output. We argue that explicitly learning the classification uncertainty as an orthogonal measure to the predicted output, is essential to account for the inherent variability characteristic of this data. Experiments were conducted on two datasets of chest radiographs of over 85,000 patients. Sample rejection based on the predicted uncertainty can significantly improve the ROC-AUC, e.g., by 8% to 0.91 with an expected rejection rate of under 25%. Eliminating training samples using uncertainty-driven bootstrapping, enables a significant increase in robustness and accuracy. In addition, we present a multi-reader study showing that the predictive uncertainty is indicative of reader errors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge