Ruhani Doda Khera
Deep Learning Predicts Cardiovascular Disease Risks from Lung Cancer Screening Low Dose Computed Tomography
Aug 16, 2020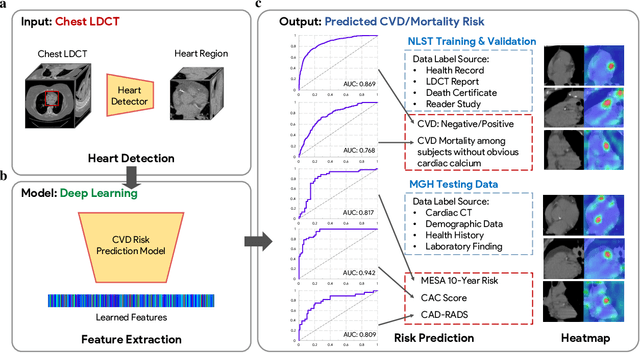

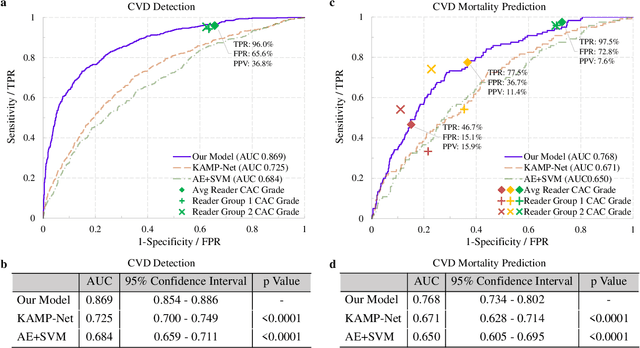

Abstract:The high risk population of cardiovascular disease (CVD) is simultaneously at high risk of lung cancer. Given the dominance of low dose computed tomography (LDCT) for lung cancer screening, the feasibility of extracting information on CVD from the same LDCT scan would add major value to patients at no additional radiation dose. However, with strong noise in LDCT images and without electrocardiogram (ECG) gating, CVD risk analysis from LDCT is highly challenging. Here we present an innovative deep learning model to address this challenge. Our deep model was trained with 30,286 LDCT volumes and achieved the state-of-the-art performance (area under the curve (AUC) of 0.869) on 2,085 National Lung Cancer Screening Trial (NLST) subjects, and effectively identified patients with high CVD mortality risks (AUC of 0.768). Our deep model was further calibrated against the clinical gold standard CVD risk scores from ECG-gated dedicated cardiac CT, including coronary artery calcification (CAC) score, CAD-RADS score and MESA 10-year CHD risk score from an independent dataset of 106 subjects. In this validation study, our model achieved AUC of 0.942, 0.809 and 0.817 for CAC, CAD-RADS and MESA scores, respectively. Our deep learning model has the potential to convert LDCT for lung cancer screening into dual-screening quantitative tool for CVD risk estimation.
Can Deep Learning Outperform Modern Commercial CT Image Reconstruction Methods?
Nov 08, 2018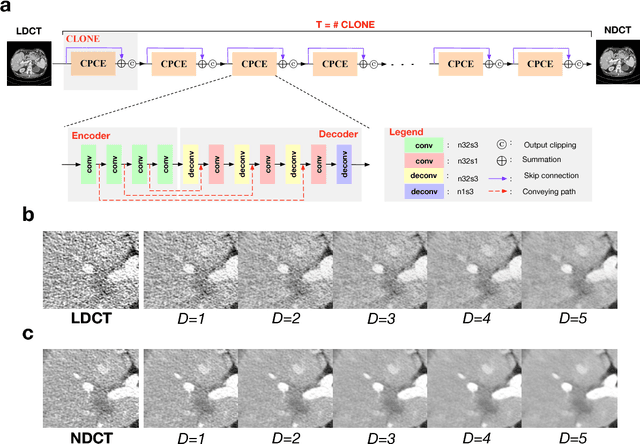
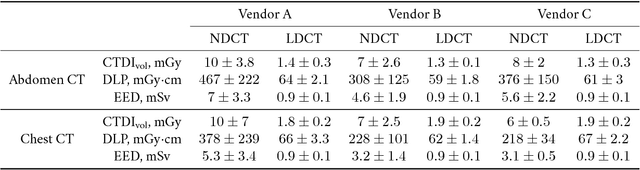
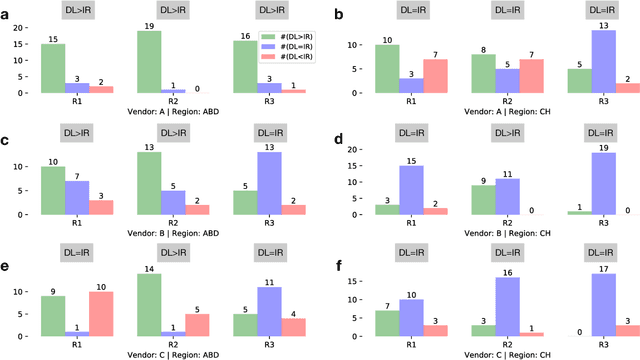

Abstract:Commercial iterative reconstruction techniques on modern CT scanners target radiation dose reduction but there are lingering concerns over their impact on image appearance and low contrast detectability. Recently, machine learning, especially deep learning, has been actively investigated for CT. Here we design a novel neural network architecture for low-dose CT (LDCT) and compare it with commercial iterative reconstruction methods used for standard of care CT. While popular neural networks are trained for end-to-end mapping, driven by big data, our novel neural network is intended for end-to-process mapping so that intermediate image targets are obtained with the associated search gradients along which the final image targets are gradually reached. This learned dynamic process allows to include radiologists in the training loop to optimize the LDCT denoising workflow in a task-specific fashion with the denoising depth as a key parameter. Our progressive denoising network was trained with the Mayo LDCT Challenge Dataset, and tested on images of the chest and abdominal regions scanned on the CT scanners made by three leading CT vendors. The best deep learning based reconstructions are systematically compared to the best iterative reconstructions in a double-blinded reader study. It is found that our deep learning approach performs either comparably or favorably in terms of noise suppression and structural fidelity, and runs orders of magnitude faster than the commercial iterative CT reconstruction algorithms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge