Mu-Han Lin
AI-Assisted Decision-Making for Clinical Assessment of Auto-Segmented Contour Quality
May 01, 2025Abstract:Purpose: This study presents a Deep Learning (DL)-based quality assessment (QA) approach for evaluating auto-generated contours (auto-contours) in radiotherapy, with emphasis on Online Adaptive Radiotherapy (OART). Leveraging Bayesian Ordinal Classification (BOC) and calibrated uncertainty thresholds, the method enables confident QA predictions without relying on ground truth contours or extensive manual labeling. Methods: We developed a BOC model to classify auto-contour quality and quantify prediction uncertainty. A calibration step was used to optimize uncertainty thresholds that meet clinical accuracy needs. The method was validated under three data scenarios: no manual labels, limited labels, and extensive labels. For rectum contours in prostate cancer, we applied geometric surrogate labels when manual labels were absent, transfer learning when limited, and direct supervision when ample labels were available. Results: The BOC model delivered robust performance across all scenarios. Fine-tuning with just 30 manual labels and calibrating with 34 subjects yielded over 90% accuracy on test data. Using the calibrated threshold, over 93% of the auto-contours' qualities were accurately predicted in over 98% of cases, reducing unnecessary manual reviews and highlighting cases needing correction. Conclusion: The proposed QA model enhances contouring efficiency in OART by reducing manual workload and enabling fast, informed clinical decisions. Through uncertainty quantification, it ensures safer, more reliable radiotherapy workflows.
Deep Learning (DL)-based Automatic Segmentation of the Internal Pudendal Artery (IPA) for Reduction of Erectile Dysfunction in Definitive Radiotherapy of Localized Prostate Cancer
Feb 03, 2023Abstract:Background and purpose: Radiation-induced erectile dysfunction (RiED) is commonly seen in prostate cancer patients. Clinical trials have been developed in multiple institutions to investigate whether dose-sparing to the internal-pudendal-arteries (IPA) will improve retention of sexual potency. The IPA is usually not considered a conventional organ-at-risk (OAR) due to segmentation difficulty. In this work, we propose a deep learning (DL)-based auto-segmentation model for the IPA that utilizes CT and MRI or CT alone as the input image modality to accommodate variation in clinical practice. Materials and methods: 86 patients with CT and MRI images and noisy IPA labels were recruited in this study. We split the data into 42/14/30 for model training, testing, and a clinical observer study, respectively. There were three major innovations in this model: 1) we designed an architecture with squeeze-and-excite blocks and modality attention for effective feature extraction and production of accurate segmentation, 2) a novel loss function was used for training the model effectively with noisy labels, and 3) modality dropout strategy was used for making the model capable of segmentation in the absence of MRI. Results: The DSC, ASD, and HD95 values for the test dataset were 62.2%, 2.54mm, and 7mm, respectively. AI segmented contours were dosimetrically equivalent to the expert physician's contours. The observer study showed that expert physicians' scored AI contours (mean=3.7) higher than inexperienced physicians' contours (mean=3.1). When inexperienced physicians started with AI contours, the score improved to 3.7. Conclusion: The proposed model achieved good quality IPA contours to improve uniformity of segmentation and to facilitate introduction of standardized IPA segmentation into clinical trials and practice.
Prior Guided Deep Difference Meta-Learner for Fast Adaptation to Stylized Segmentation
Nov 19, 2022Abstract:When a pre-trained general auto-segmentation model is deployed at a new institution, a support framework in the proposed Prior-guided DDL network will learn the systematic difference between the model predictions and the final contours revised and approved by clinicians for an initial group of patients. The learned style feature differences are concatenated with the new patients (query) features and then decoded to get the style-adapted segmentations. The model is independent of practice styles and anatomical structures. It meta-learns with simulated style differences and does not need to be exposed to any real clinical stylized structures during training. Once trained on the simulated data, it can be deployed for clinical use to adapt to new practice styles and new anatomical structures without further training. To show the proof of concept, we tested the Prior-guided DDL network on six different practice style variations for three different anatomical structures. Pre-trained segmentation models were adapted from post-operative clinical target volume (CTV) segmentation to segment CTVstyle1, CTVstyle2, and CTVstyle3, from parotid gland segmentation to segment Parotidsuperficial, and from rectum segmentation to segment Rectumsuperior and Rectumposterior. The mode performance was quantified with Dice Similarity Coefficient (DSC). With adaptation based on only the first three patients, the average DSCs were improved from 78.6, 71.9, 63.0, 52.2, 46.3 and 69.6 to 84.4, 77.8, 73.0, 77.8, 70.5, 68.1, for CTVstyle1, CTVstyle2, and CTVstyle3, Parotidsuperficial, Rectumsuperior, and Rectumposterior, respectively, showing the great potential of the Priorguided DDL network for a fast and effortless adaptation to new practice styles
Performance Deterioration of Deep Learning Models after Clinical Deployment: A Case Study with Auto-segmentation for Definitive Prostate Cancer Radiotherapy
Oct 11, 2022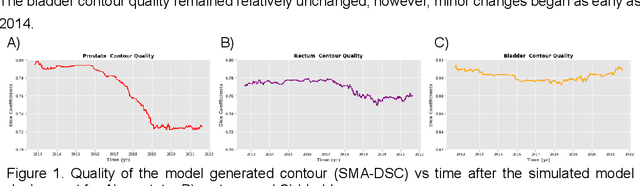
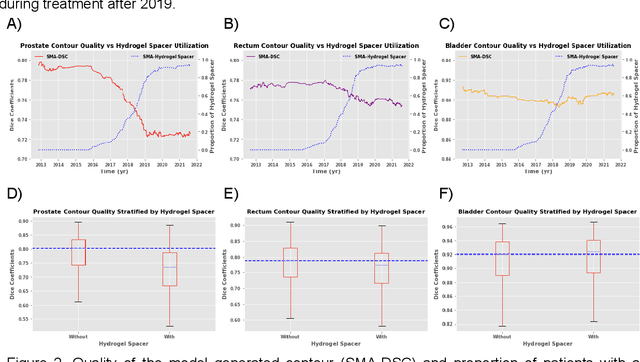
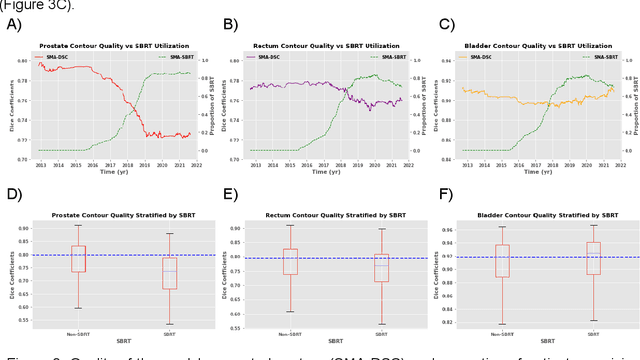
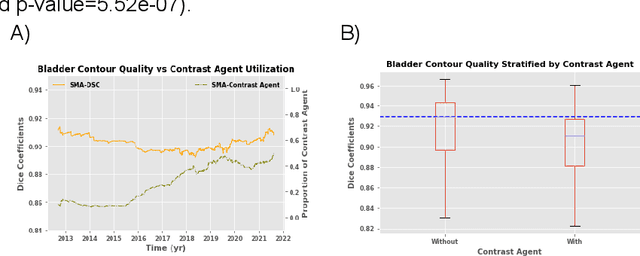
Abstract:In the past decade, deep learning (DL)-based artificial intelligence (AI) has witnessed unprecedented success and has led to much excitement in medicine. However, many successful models have not been implemented in the clinic predominantly due to concerns regarding the lack of interpretability and generalizability in both spatial and temporal domains. In this work, we used a DL-based auto segmentation model for intact prostate patients to observe any temporal performance changes and then correlate them to possible explanatory variables. We retrospectively simulated the clinical implementation of our DL model to investigate temporal performance trends. Our cohort included 912 patients with prostate cancer treated with definitive radiotherapy from January 2006 to August 2021 at the University of Texas Southwestern Medical Center (UTSW). We trained a U-Net-based DL auto segmentation model on the data collected before 2012 and tested it on data collected from 2012 to 2021 to simulate the clinical deployment of the trained model starting in 2012. We visualize the trends using a simple moving average curve and used ANOVA and t-test to investigate the impact of various clinical factors. The prostate and rectum contour quality decreased rapidly after 2016-2017. Stereotactic body radiotherapy (SBRT) and hydrogel spacer use were significantly associated with prostate contour quality (p=5.6e-12 and 0.002, respectively). SBRT and physicians' styles are significantly associated with the rectum contour quality (p=0.0005 and 0.02, respectively). Only the presence of contrast within the bladder significantly affected the bladder contour quality (p=1.6e-7). We showed that DL model performance decreased over time in concordance with changes in clinical practice patterns and changes in clinical personnel.
Registration-Guided Deep Learning Image Segmentation for Cone Beam CT-based Online Adaptive Radiotherapy
Aug 19, 2021



Abstract:Adaptive radiotherapy (ART), especially online ART, effectively accounts for positioning errors and anatomical changes. One key component of online ART is accurately and efficiently delineating organs at risk (OARs) and targets on online images, such as CBCT, to meet the online demands of plan evaluation and adaptation. Deep learning (DL)-based automatic segmentation has gained great success in segmenting planning CT, but its applications to CBCT yielded inferior results due to the low image quality and limited available contour labels for training. To overcome these obstacles to online CBCT segmentation, we propose a registration-guided DL (RgDL) segmentation framework that integrates image registration algorithms and DL segmentation models. The registration algorithm generates initial contours, which were used as guidance by DL model to obtain accurate final segmentations. We had two implementations the proposed framework--Rig-RgDL (Rig for rigid body) and Def-RgDL (Def for deformable)--with rigid body (RB) registration or deformable image registration (DIR) as the registration algorithm respectively and U-Net as DL model architecture. The two implementations of RgDL framework were trained and evaluated on seven OARs in an institutional clinical Head and Neck (HN) dataset. Compared to the baseline approaches using the registration or the DL alone, RgDL achieved more accurate segmentation, as measured by higher mean Dice similarity coefficients (DSC) and other distance-based metrics. Rig-RgDL achieved a DSC of 84.5% on seven OARs on average, higher than RB or DL alone by 4.5% and 4.7%. The DSC of Def-RgDL is 86.5%, higher than DIR or DL alone by 2.4% and 6.7%. The inference time took by the DL model to generate final segmentations of seven OARs is less than one second in RgDL. The resulting segmentation accuracy and efficiency show the promise of applying RgDL framework for online ART.
A Proof-of-Concept Study of Artificial Intelligence Assisted Contour Revision
Jul 28, 2021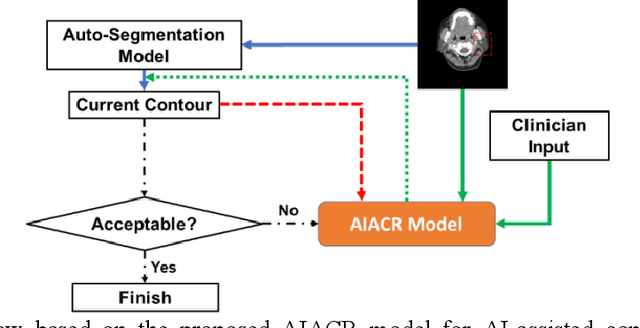

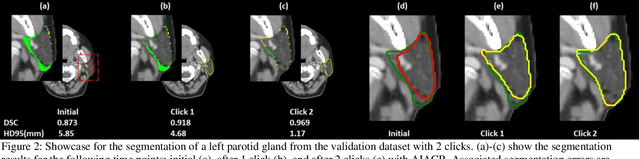
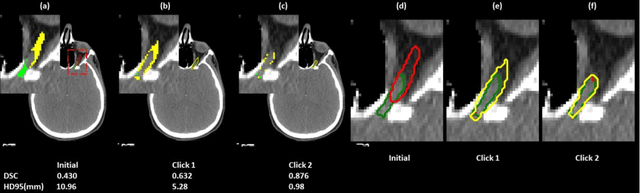
Abstract:Automatic segmentation of anatomical structures is critical for many medical applications. However, the results are not always clinically acceptable and require tedious manual revision. Here, we present a novel concept called artificial intelligence assisted contour revision (AIACR) and demonstrate its feasibility. The proposed clinical workflow of AIACR is as follows given an initial contour that requires a clinicians revision, the clinician indicates where a large revision is needed, and a trained deep learning (DL) model takes this input to update the contour. This process repeats until a clinically acceptable contour is achieved. The DL model is designed to minimize the clinicians input at each iteration and to minimize the number of iterations needed to reach acceptance. In this proof-of-concept study, we demonstrated the concept on 2D axial images of three head-and-neck cancer datasets, with the clinicians input at each iteration being one mouse click on the desired location of the contour segment. The performance of the model is quantified with Dice Similarity Coefficient (DSC) and 95th percentile of Hausdorff Distance (HD95). The average DSC/HD95 (mm) of the auto-generated initial contours were 0.82/4.3, 0.73/5.6 and 0.67/11.4 for three datasets, which were improved to 0.91/2.1, 0.86/2.4 and 0.86/4.7 with three mouse clicks, respectively. Each DL-based contour update requires around 20 ms. We proposed a novel AIACR concept that uses DL models to assist clinicians in revising contours in an efficient and effective way, and we demonstrated its feasibility by using 2D axial CT images from three head-and-neck cancer datasets.
Site-Agnostic 3D Dose Distribution Prediction with Deep Learning Neural Networks
Jun 15, 2021
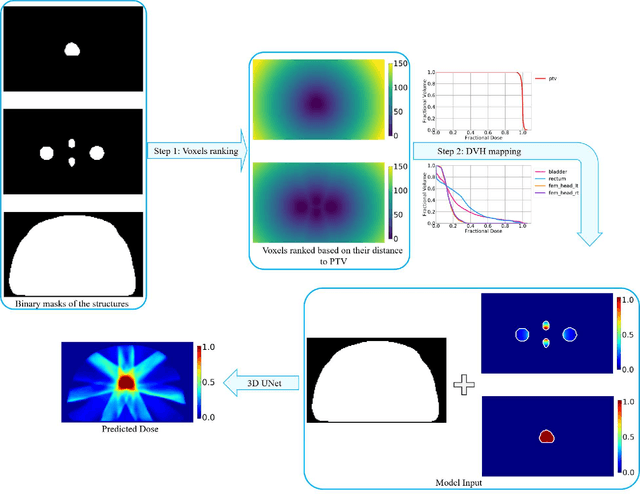
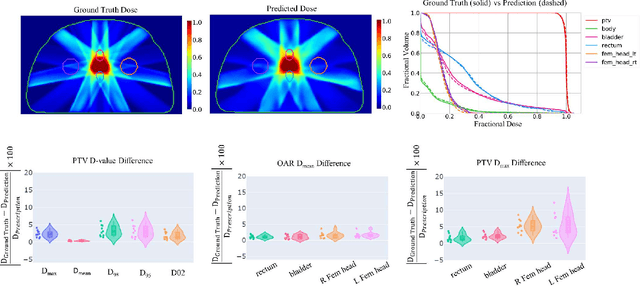
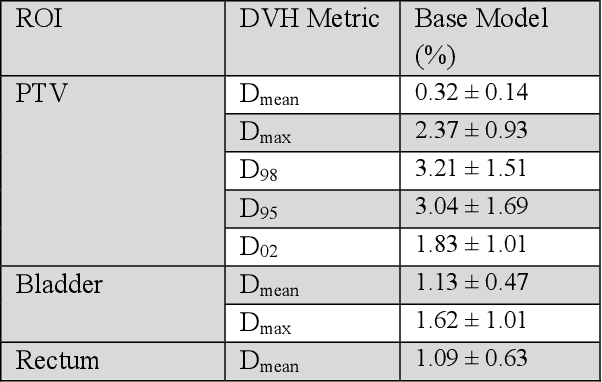
Abstract:Typically, the current dose prediction models are limited to small amounts of data and require re-training for a specific site, often leading to suboptimal performance. We propose a site-agnostic, 3D dose distribution prediction model using deep learning that can leverage data from any treatment site, thus increasing the total data available to train the model. Applying our proposed model to a new target treatment site requires only a brief fine-tuning of the model to the new data and involves no modifications to the model input channels or its parameters. Thus, it can be efficiently adapted to a different treatment site, even with a small training dataset.
Dosimetric impact of physician style variations in contouring CTV for post-operative prostate cancer: A deep learning based simulation study
Feb 01, 2021
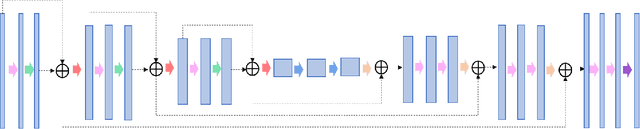
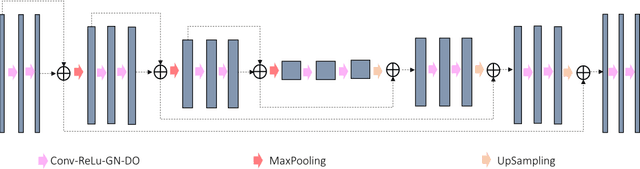
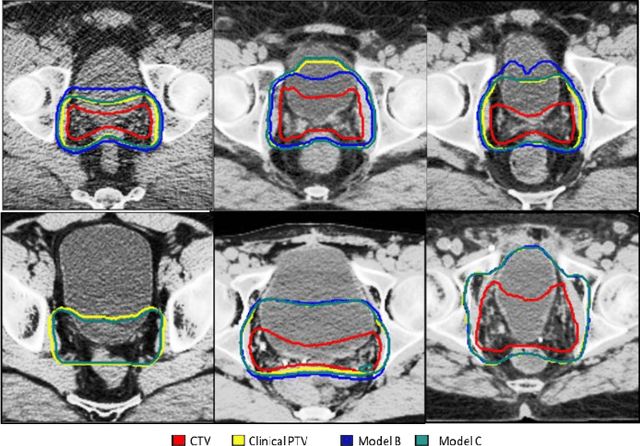
Abstract:In tumor segmentation, inter-observer variation is acknowledged to be a significant problem. This is even more significant in clinical target volume (CTV) segmentation, specifically, in post-operative settings, where a gross tumor does not exist. In this scenario, CTV is not an anatomically established structure but rather one determined by the physician based on the clinical guideline used, the preferred trade off between tumor control and toxicity, their experience, training background etc... This results in high inter-observer variability between physicians. Inter-observer variability has been considered an issue, however its dosimetric consequence is still unclear, due to the absence of multiple physician CTV contours for each patient and the significant amount of time required for dose planning. In this study, we analyze the impact that these physician stylistic variations have on organs-at-risk (OAR) dose by simulating the clinical workflow using deep learning. For a given patient previously treated by one physician, we use DL-based tools to simulate how other physicians would contour the CTV and how the corresponding dose distributions should look like for this patient. To simulate multiple physician styles, we use a previously developed in-house CTV segmentation model that can produce physician style-aware segmentations. The corresponding dose distribution is predicted using another in-house deep learning tool, which, averaging across all structures, is capable of predicting dose within 3% of the prescription dose on the test data. For every test patient, four different physician-style CTVs are considered and four different dose distributions are analyzed. OAR dose metrics are compared, showing that even though physician style variations results in organs getting different doses, all the important dose metrics except Maximum Dose point are within the clinically acceptable limit.
Using Monte Carlo dropout and bootstrap aggregation for uncertainty estimation in radiation therapy dose prediction with deep learning neural networks
Nov 01, 2020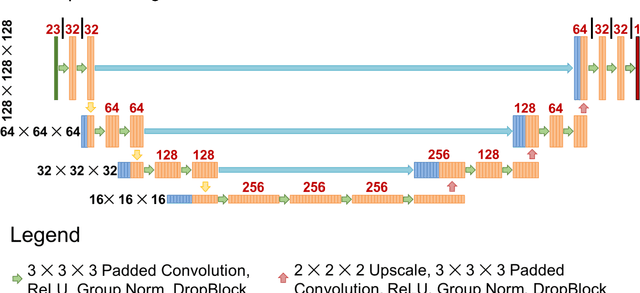
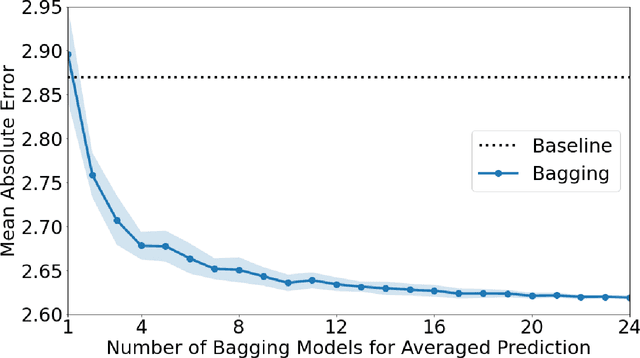
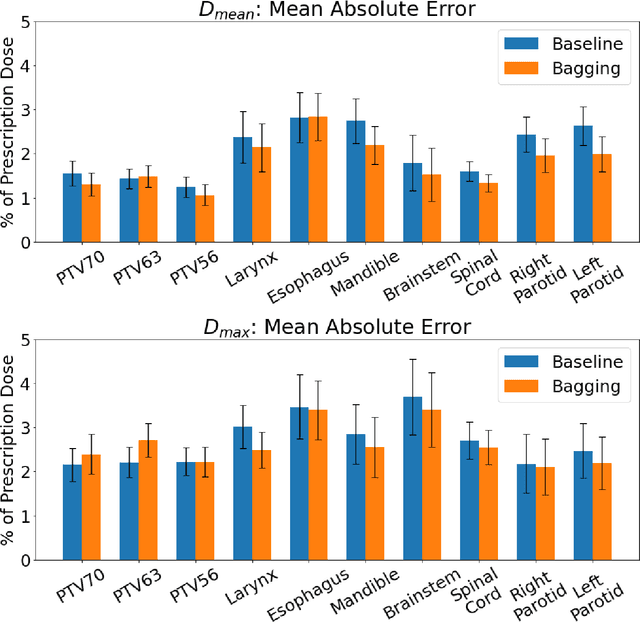
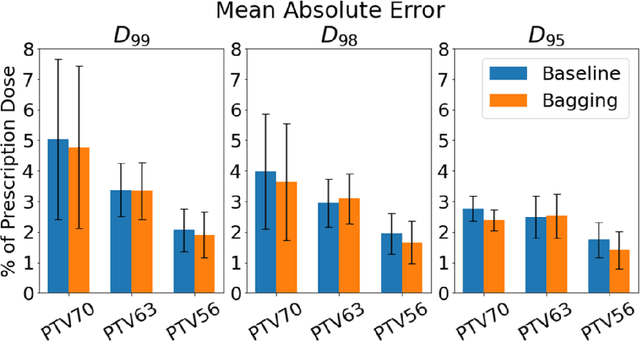
Abstract:Recently, artificial intelligence technologies and algorithms have become a major focus for advancements in treatment planning for radiation therapy. As these are starting to become incorporated into the clinical workflow, a major concern from clinicians is not whether the model is accurate, but whether the model can express to a human operator when it does not know if its answer is correct. We propose to use Monte Carlo dropout (MCDO) and the bootstrap aggregation (bagging) technique on deep learning models to produce uncertainty estimations for radiation therapy dose prediction. We show that both models are capable of generating a reasonable uncertainty map, and, with our proposed scaling technique, creating interpretable uncertainties and bounds on the prediction and any relevant metrics. Performance-wise, bagging provides statistically significant reduced loss value and errors in most of the metrics investigated in this study. The addition of bagging was able to further reduce errors by another 0.34% for Dmean and 0.19% for Dmax, on average, when compared to the baseline framework. Overall, the bagging framework provided significantly lower MAE of 2.62, as opposed to the baseline framework's MAE of 2.87. The usefulness of bagging, from solely a performance standpoint, does highly depend on the problem and the acceptable predictive error, and its high upfront computational cost during training should be factored in to deciding whether it is advantageous to use it. In terms of deployment with uncertainty estimations turned on, both frameworks offer the same performance time of about 12 seconds. As an ensemble-based metaheuristic, bagging can be used with existing machine learning architectures to improve stability and performance, and MCDO can be applied to any deep learning models that have dropout as part of their architecture.
Dose Prediction with Deep Learning for Prostate Cancer Radiation Therapy: Model Adaptation to Different Treatment Planning Practices
Jun 30, 2020



Abstract:This work aims to study the generalizability of a pre-developed deep learning (DL) dose prediction model for volumetric modulated arc therapy (VMAT) for prostate cancer and to adapt the model to three different internal treatment planning styles and one external institution planning style. We built the source model with planning data from 108 patients previously treated with VMAT for prostate cancer. For the transfer learning, we selected patient cases planned with three different styles from the same institution and one style from a different institution to adapt the source model to four target models. We compared the dose distributions predicted by the source model and the target models with the clinical dose predictions and quantified the improvement in the prediction quality for the target models over the source model using the Dice similarity coefficients (DSC) of 10% to 100% isodose volumes and the dose-volume-histogram (DVH) parameters of the planning target volume and the organs-at-risk. The source model accurately predicts dose distributions for plans generated in the same source style but performs sub-optimally for the three internal and one external target styles, with the mean DSC ranging between 0.81-0.94 and 0.82-0.91 for the internal and the external styles, respectively. With transfer learning, the target model predictions improved the mean DSC to 0.88-0.95 and 0.92-0.96 for the internal and the external styles, respectively. Target model predictions significantly improved the accuracy of the DVH parameter predictions to within 1.6%. We demonstrated model generalizability for DL-based dose prediction and the feasibility of using transfer learning to solve this problem. With 14-29 cases per style, we successfully adapted the source model into several different practice styles. This indicates a realistic way to widespread clinical implementation of DL-based dose prediction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge