Xinran Zhong
Deep Learning (DL)-based Automatic Segmentation of the Internal Pudendal Artery (IPA) for Reduction of Erectile Dysfunction in Definitive Radiotherapy of Localized Prostate Cancer
Feb 03, 2023Abstract:Background and purpose: Radiation-induced erectile dysfunction (RiED) is commonly seen in prostate cancer patients. Clinical trials have been developed in multiple institutions to investigate whether dose-sparing to the internal-pudendal-arteries (IPA) will improve retention of sexual potency. The IPA is usually not considered a conventional organ-at-risk (OAR) due to segmentation difficulty. In this work, we propose a deep learning (DL)-based auto-segmentation model for the IPA that utilizes CT and MRI or CT alone as the input image modality to accommodate variation in clinical practice. Materials and methods: 86 patients with CT and MRI images and noisy IPA labels were recruited in this study. We split the data into 42/14/30 for model training, testing, and a clinical observer study, respectively. There were three major innovations in this model: 1) we designed an architecture with squeeze-and-excite blocks and modality attention for effective feature extraction and production of accurate segmentation, 2) a novel loss function was used for training the model effectively with noisy labels, and 3) modality dropout strategy was used for making the model capable of segmentation in the absence of MRI. Results: The DSC, ASD, and HD95 values for the test dataset were 62.2%, 2.54mm, and 7mm, respectively. AI segmented contours were dosimetrically equivalent to the expert physician's contours. The observer study showed that expert physicians' scored AI contours (mean=3.7) higher than inexperienced physicians' contours (mean=3.1). When inexperienced physicians started with AI contours, the score improved to 3.7. Conclusion: The proposed model achieved good quality IPA contours to improve uniformity of segmentation and to facilitate introduction of standardized IPA segmentation into clinical trials and practice.
Site-Agnostic 3D Dose Distribution Prediction with Deep Learning Neural Networks
Jun 15, 2021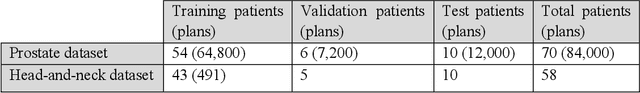
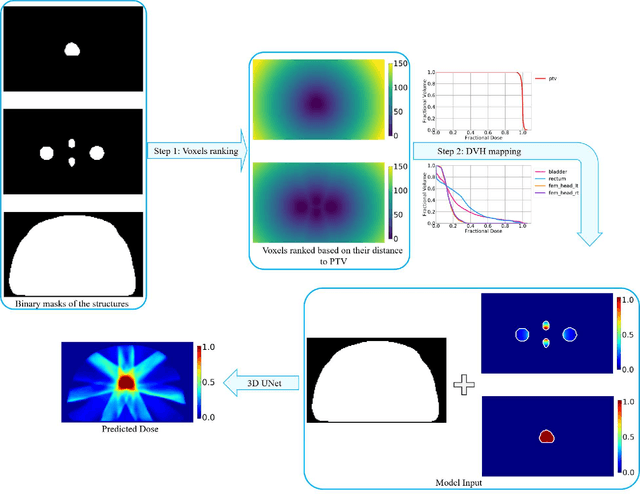
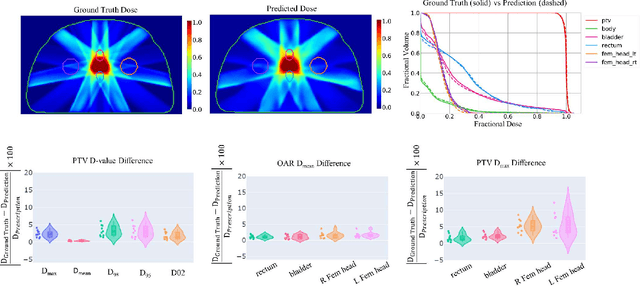
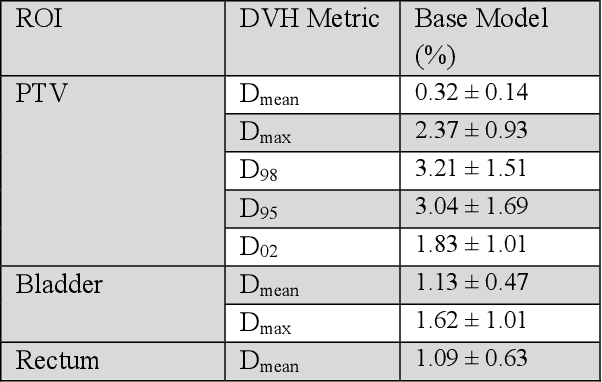
Abstract:Typically, the current dose prediction models are limited to small amounts of data and require re-training for a specific site, often leading to suboptimal performance. We propose a site-agnostic, 3D dose distribution prediction model using deep learning that can leverage data from any treatment site, thus increasing the total data available to train the model. Applying our proposed model to a new target treatment site requires only a brief fine-tuning of the model to the new data and involves no modifications to the model input channels or its parameters. Thus, it can be efficiently adapted to a different treatment site, even with a small training dataset.
Automatic Prostate Zonal Segmentation Using Fully Convolutional Network with Feature Pyramid Attention
Oct 31, 2019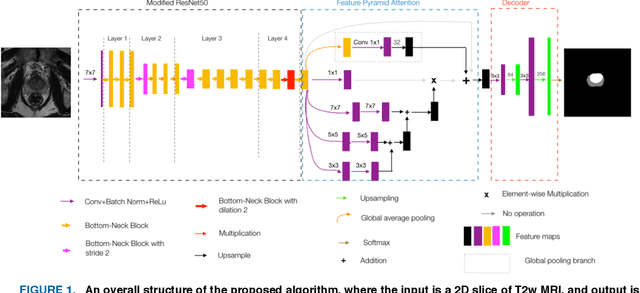
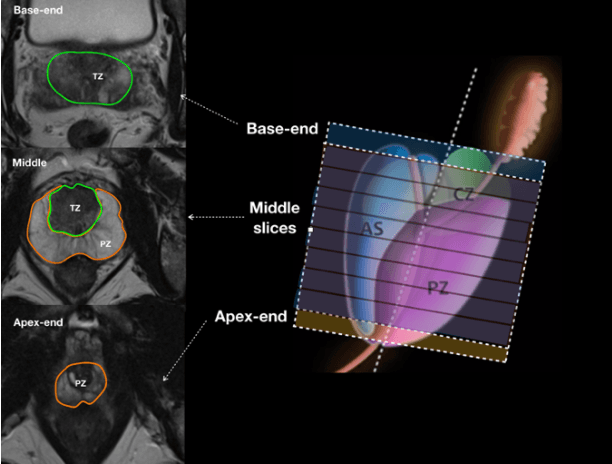
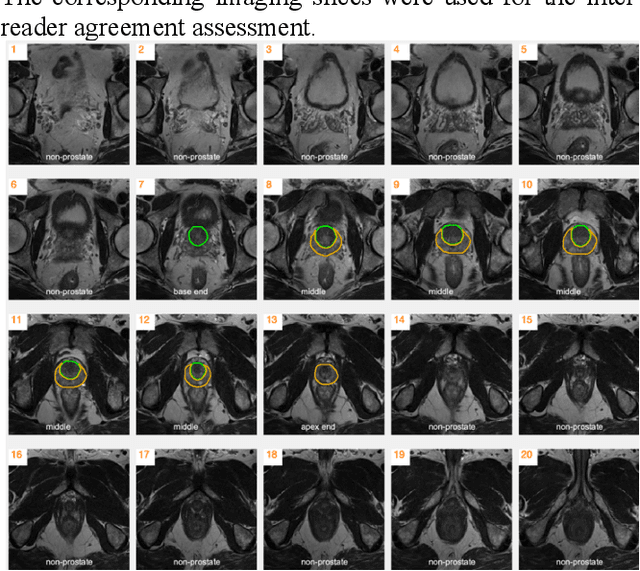
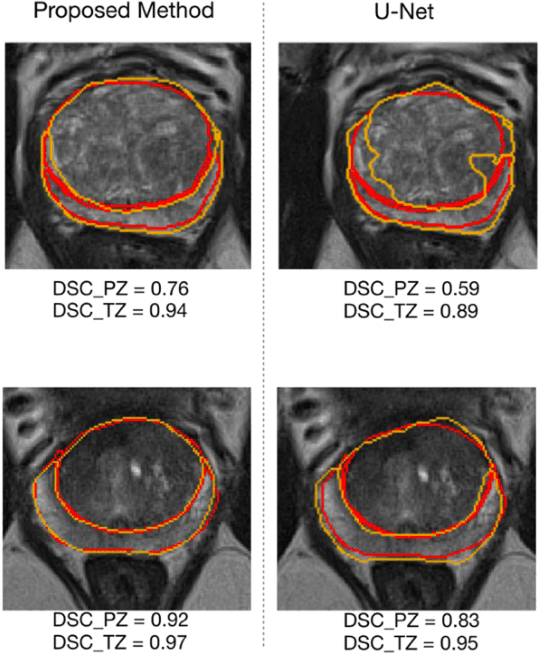
Abstract:Our main objective is to develop a novel deep learning-based algorithm for automatic segmentation of prostate zone and to evaluate the proposed algorithm on an additional independent testing data in comparison with inter-reader consistency between two experts. With IRB approval and HIPAA compliance, we designed a novel convolutional neural network (CNN) for automatic segmentation of the prostatic transition zone (TZ) and peripheral zone (PZ) on T2-weighted (T2w) MRI. The total study cohort included 359 patients from two sources; 313 from a deidentified publicly available dataset (SPIE-AAPM-NCI PROSTATEX challenge) and 46 from a large U.S. tertiary referral center with 3T MRI (external testing dataset (ETD)). The TZ and PZ contours were manually annotated by research fellows, supervised by genitourinary (GU) radiologists. The model was developed using 250 patients and tested internally using the remaining 63 patients from the PROSTATEX (internal testing dataset (ITD)) and tested again (n=46) externally using the ETD. The Dice Similarity Coefficient (DSC) was used to evaluate the segmentation performance. DSCs for PZ and TZ were 0.74 and 0.86 in the ITD respectively. In the ETD, DSCs for PZ and TZ were 0.74 and 0.792, respectively. The inter-reader consistency (Expert 2 vs. Expert 1) were 0.71 (PZ) and 0.75 (TZ). This novel DL algorithm enabled automatic segmentation of PZ and TZ with high accuracy on both ITD and ETD without a performance difference for PZ and less than 10% TZ difference. In the ETD, the proposed method can be comparable to experts in the segmentation of prostate zones.
Prostate cancer inference via weakly-supervised learning using a large collection of negative MRI
Oct 05, 2019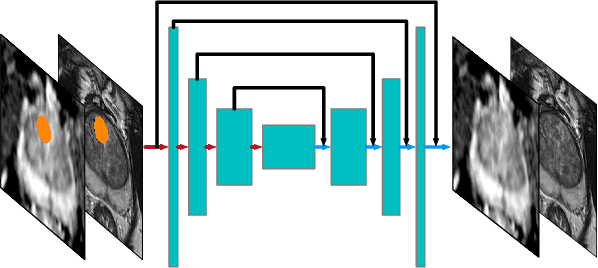
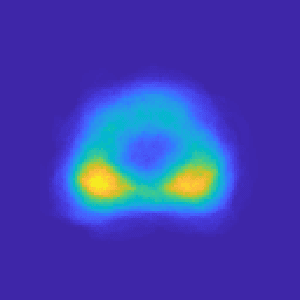
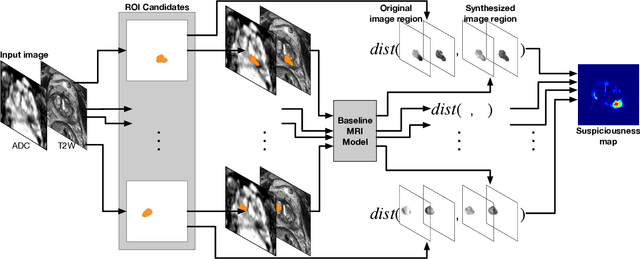
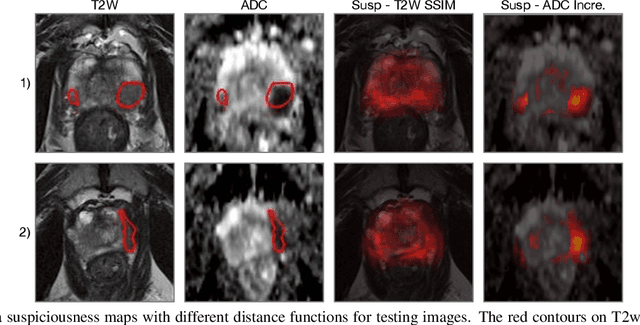
Abstract:Recent advances in medical imaging techniques have led to significant improvements in the management of prostate cancer (PCa). In particular, multi-parametric MRI (mp-MRI) continues to gain clinical acceptance as the preferred imaging technique for non-invasive detection and grading of PCa. However, the machine learning-based diagnosis systems for PCa are often constrained by the limited access to accurate lesion ground truth annotations for training. The performance of the machine learning system is highly dependable on both quality and quantity of lesion annotations associated with histopathologic findings, resulting in limited scalability and clinical validation. Here, we propose the baseline MRI model to alternatively learn the appearance of mp-MRI using radiology-confirmed negative MRI cases via weakly supervised learning. Since PCa lesions are case-specific and highly heterogeneous, it is assumed to be challenging to synthesize PCa lesions using the baseline MRI model, while it would be relatively easier to synthesize the normal appearance in mp-MRI. We then utilize the baseline MRI model to infer the pixel-wise suspiciousness of PCa by comparing the original and synthesized MRI with two distance functions. We trained and validated the baseline MRI model using 1,145 negative prostate mp-MRI scans. For evaluation, we used separated 232 mp-MRI scans, consisting of both positive and negative MRI cases. The 116 positive MRI scans were annotated by radiologists, confirmed with post-surgical whole-gland specimens. The suspiciousness map was evaluated by receiver operating characteristic (ROC) analysis for PCa lesions versus non-PCa regions classification and free-response receiver operating characteristic (FROC) analysis for PCa localization. Our proposed method achieved 0.84 area under the ROC curve and 77.0% sensitivity at one false positive per patient in FROC analysis.
Deep Learning-based Radiomic Features for Improving Neoadjuvant Chemoradiation Response Prediction in Locally Advanced Rectal Cancer
Sep 09, 2019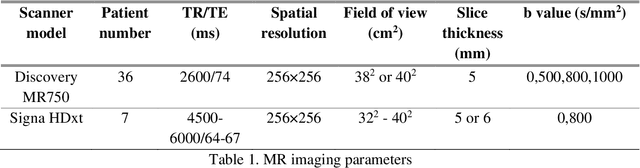
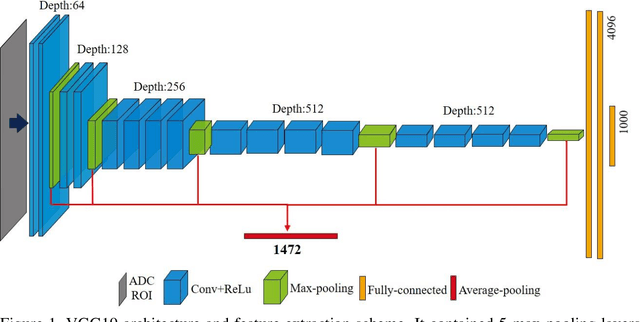
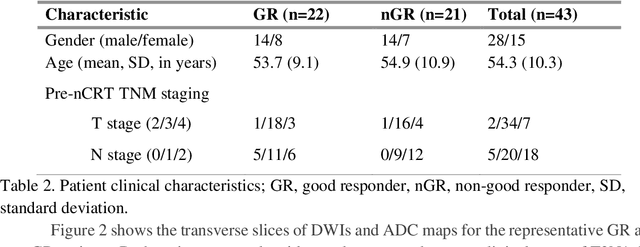
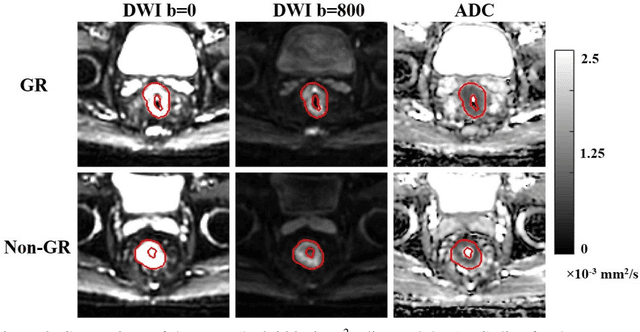
Abstract:Radiomic features achieve promising results in cancer diagnosis, treatment response prediction, and survival prediction. Our goal is to compare the handcrafted (explicitly designed) and deep learning (DL)-based radiomic features extracted from pre-treatment diffusion-weighted magnetic resonance images (DWIs) for predicting neoadjuvant chemoradiation treatment (nCRT) response in patients with locally advanced rectal cancer (LARC). 43 patients receiving nCRT were included. All patients underwent DWIs before nCRT and total mesorectal excision surgery 6-12 weeks after completion of nCRT. Gross tumor volume (GTV) contours were drawn by an experienced radiation oncologist on DWIs. The patient-cohort was split into the responder group (n=22) and the non-responder group (n=21) based on the post-nCRT response assessed by postoperative pathology, MRI or colonoscopy. Handcrafted and DL-based features were extracted from the apparent diffusion coefficient (ADC) map of the DWI using conventional computer-aided diagnosis methods and a pre-trained convolution neural network, respectively. Least absolute shrinkage and selection operator (LASSO)-logistic regression models were constructed using extracted features for predicting treatment response. The model performance was evaluated with repeated 20 times stratified 4-fold cross-validation using receiver operating characteristic (ROC) curves and compared using the corrected resampled t-test. The model built with handcrafted features achieved the mean area under the ROC curve (AUC) of 0.64, while the one built with DL-based features yielded the mean AUC of 0.73. The corrected resampled t-test on AUC showed P-value < 0.05. DL-based features extracted from pre-treatment DWIs achieved significantly better classification performance compared with handcrafted features for predicting nCRT response in patients with LARC.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge