Steven Raman
Federated Learning with Research Prototypes for Multi-Center MRI-based Detection of Prostate Cancer with Diverse Histopathology
Jun 11, 2022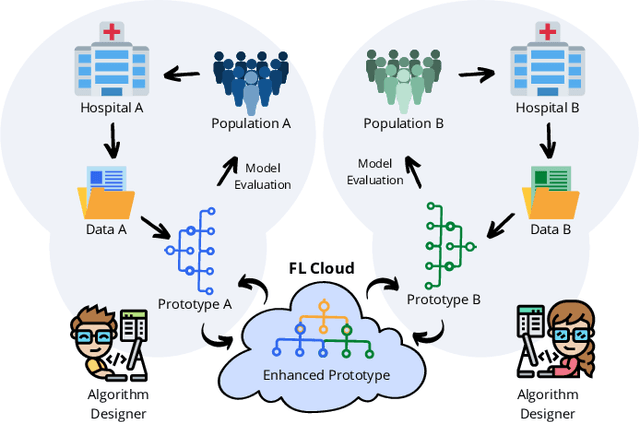
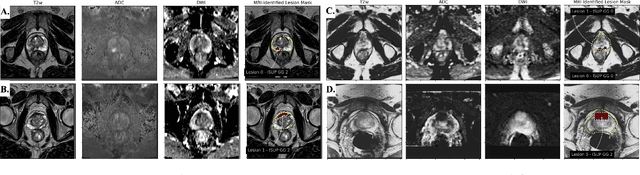
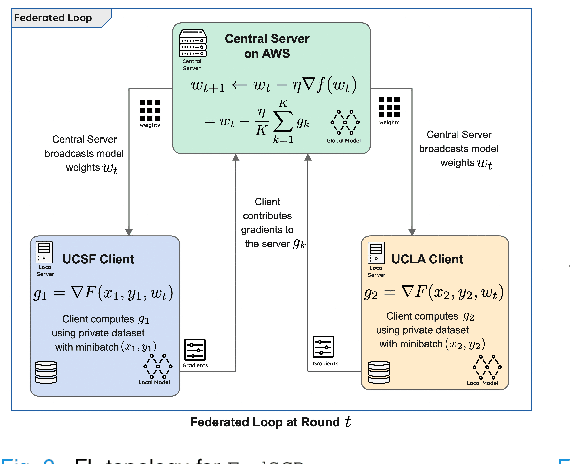
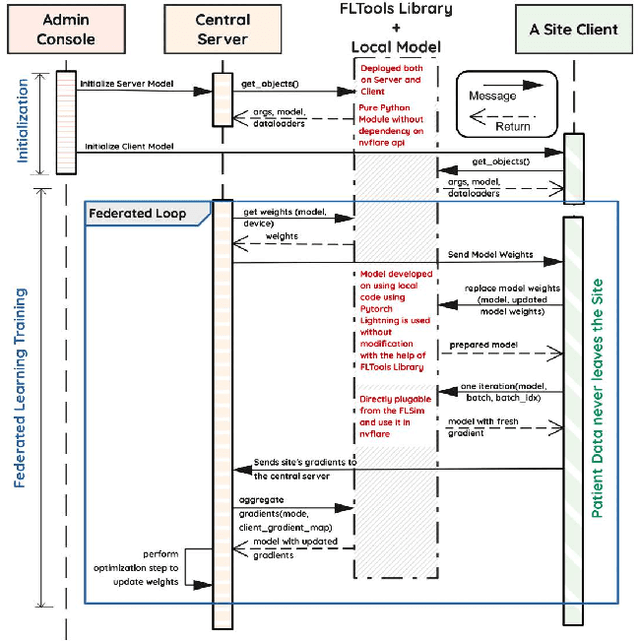
Abstract:Early prostate cancer detection and staging from MRI are extremely challenging tasks for both radiologists and deep learning algorithms, but the potential to learn from large and diverse datasets remains a promising avenue to increase their generalization capability both within- and across clinics. To enable this for prototype-stage algorithms, where the majority of existing research remains, in this paper we introduce a flexible federated learning framework for cross-site training, validation, and evaluation of deep prostate cancer detection algorithms. Our approach utilizes an abstracted representation of the model architecture and data, which allows unpolished prototype deep learning models to be trained without modification using the NVFlare federated learning framework. Our results show increases in prostate cancer detection and classification accuracy using a specialized neural network model and diverse prostate biopsy data collected at two University of California research hospitals, demonstrating the efficacy of our approach in adapting to different datasets and improving MR-biomarker discovery. We open-source our FLtools system, which can be easily adapted to other deep learning projects for medical imaging.
Automatic Prostate Zonal Segmentation Using Fully Convolutional Network with Feature Pyramid Attention
Oct 31, 2019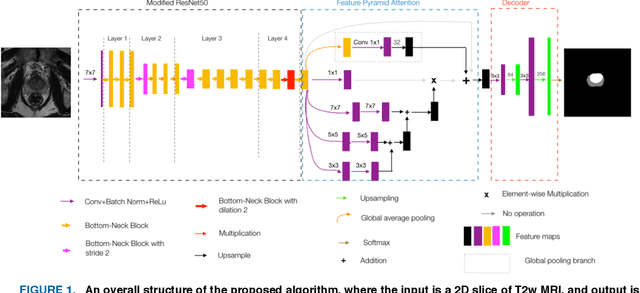
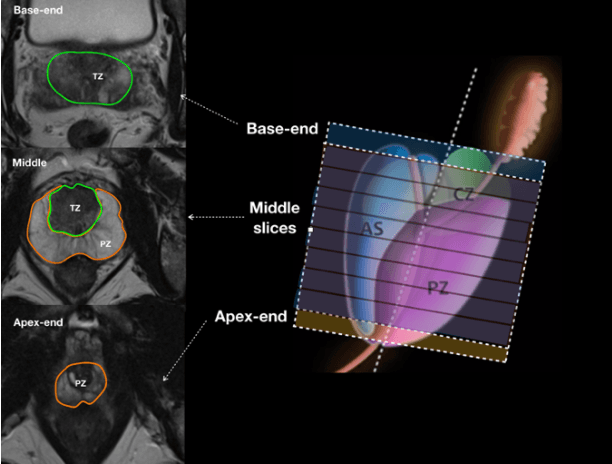
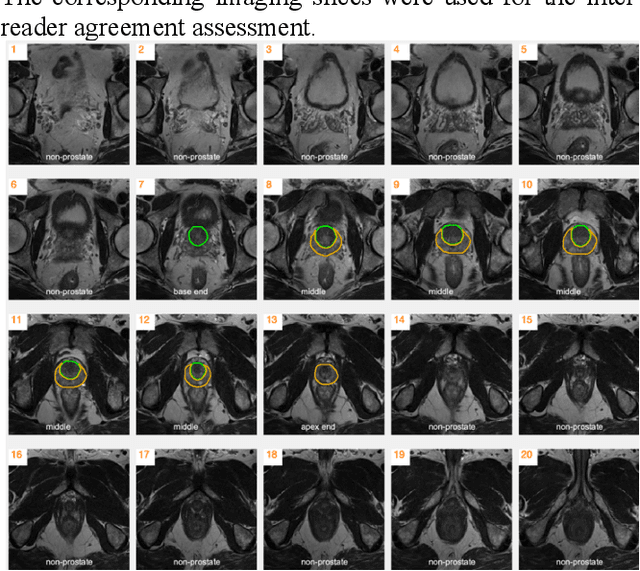
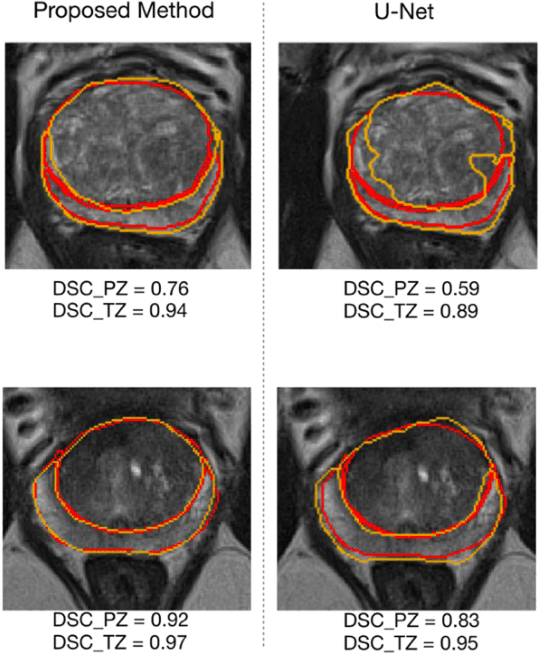
Abstract:Our main objective is to develop a novel deep learning-based algorithm for automatic segmentation of prostate zone and to evaluate the proposed algorithm on an additional independent testing data in comparison with inter-reader consistency between two experts. With IRB approval and HIPAA compliance, we designed a novel convolutional neural network (CNN) for automatic segmentation of the prostatic transition zone (TZ) and peripheral zone (PZ) on T2-weighted (T2w) MRI. The total study cohort included 359 patients from two sources; 313 from a deidentified publicly available dataset (SPIE-AAPM-NCI PROSTATEX challenge) and 46 from a large U.S. tertiary referral center with 3T MRI (external testing dataset (ETD)). The TZ and PZ contours were manually annotated by research fellows, supervised by genitourinary (GU) radiologists. The model was developed using 250 patients and tested internally using the remaining 63 patients from the PROSTATEX (internal testing dataset (ITD)) and tested again (n=46) externally using the ETD. The Dice Similarity Coefficient (DSC) was used to evaluate the segmentation performance. DSCs for PZ and TZ were 0.74 and 0.86 in the ITD respectively. In the ETD, DSCs for PZ and TZ were 0.74 and 0.792, respectively. The inter-reader consistency (Expert 2 vs. Expert 1) were 0.71 (PZ) and 0.75 (TZ). This novel DL algorithm enabled automatic segmentation of PZ and TZ with high accuracy on both ITD and ETD without a performance difference for PZ and less than 10% TZ difference. In the ETD, the proposed method can be comparable to experts in the segmentation of prostate zones.
Prostate cancer inference via weakly-supervised learning using a large collection of negative MRI
Oct 05, 2019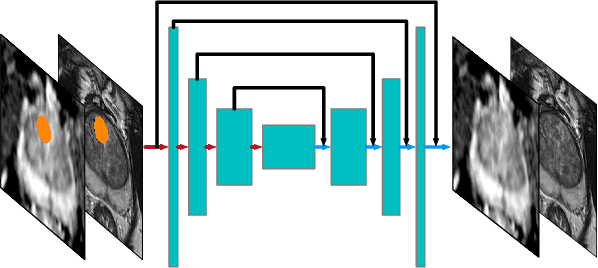
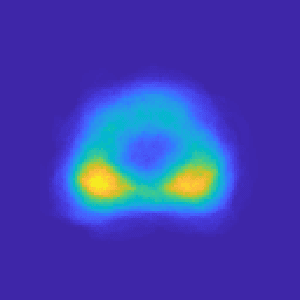
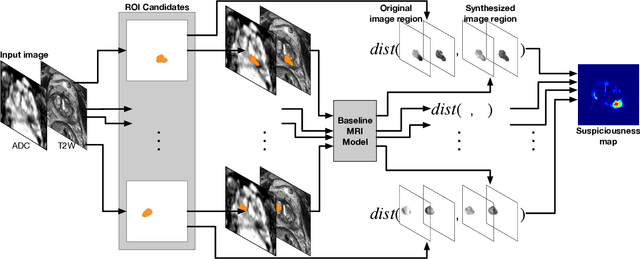
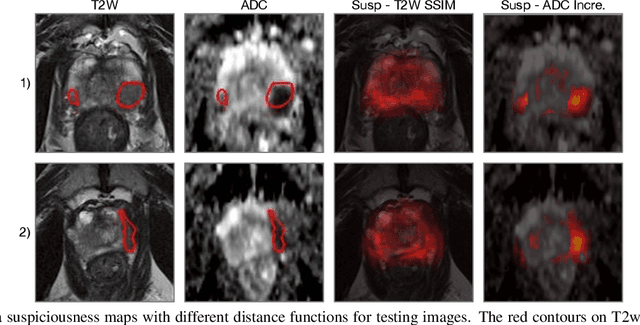
Abstract:Recent advances in medical imaging techniques have led to significant improvements in the management of prostate cancer (PCa). In particular, multi-parametric MRI (mp-MRI) continues to gain clinical acceptance as the preferred imaging technique for non-invasive detection and grading of PCa. However, the machine learning-based diagnosis systems for PCa are often constrained by the limited access to accurate lesion ground truth annotations for training. The performance of the machine learning system is highly dependable on both quality and quantity of lesion annotations associated with histopathologic findings, resulting in limited scalability and clinical validation. Here, we propose the baseline MRI model to alternatively learn the appearance of mp-MRI using radiology-confirmed negative MRI cases via weakly supervised learning. Since PCa lesions are case-specific and highly heterogeneous, it is assumed to be challenging to synthesize PCa lesions using the baseline MRI model, while it would be relatively easier to synthesize the normal appearance in mp-MRI. We then utilize the baseline MRI model to infer the pixel-wise suspiciousness of PCa by comparing the original and synthesized MRI with two distance functions. We trained and validated the baseline MRI model using 1,145 negative prostate mp-MRI scans. For evaluation, we used separated 232 mp-MRI scans, consisting of both positive and negative MRI cases. The 116 positive MRI scans were annotated by radiologists, confirmed with post-surgical whole-gland specimens. The suspiciousness map was evaluated by receiver operating characteristic (ROC) analysis for PCa lesions versus non-PCa regions classification and free-response receiver operating characteristic (FROC) analysis for PCa localization. Our proposed method achieved 0.84 area under the ROC curve and 77.0% sensitivity at one false positive per patient in FROC analysis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge