Gyanendra Bohara
Using Monte Carlo dropout and bootstrap aggregation for uncertainty estimation in radiation therapy dose prediction with deep learning neural networks
Nov 01, 2020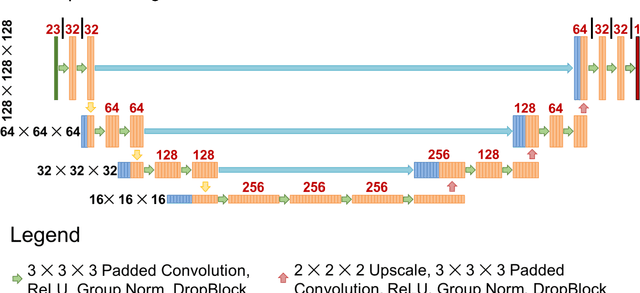
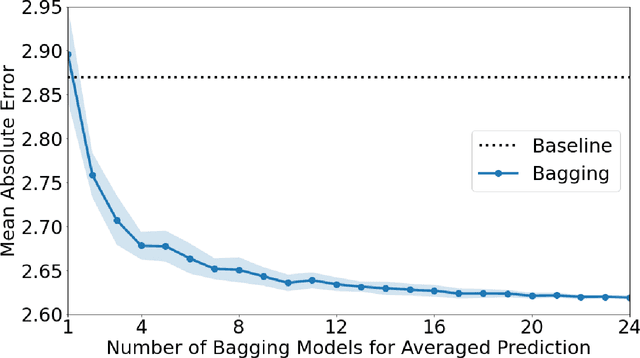
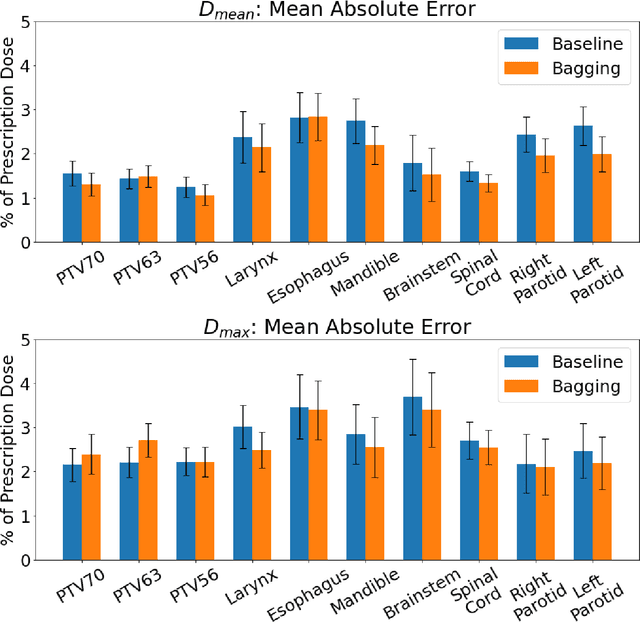
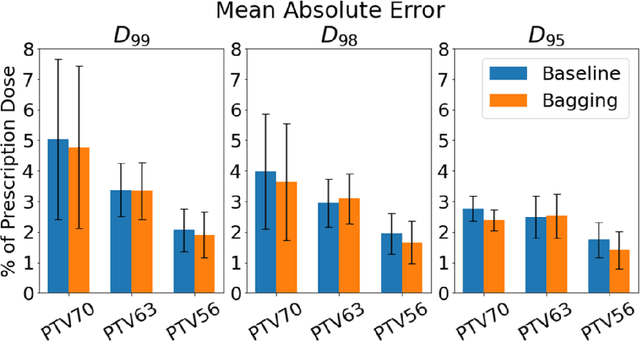
Abstract:Recently, artificial intelligence technologies and algorithms have become a major focus for advancements in treatment planning for radiation therapy. As these are starting to become incorporated into the clinical workflow, a major concern from clinicians is not whether the model is accurate, but whether the model can express to a human operator when it does not know if its answer is correct. We propose to use Monte Carlo dropout (MCDO) and the bootstrap aggregation (bagging) technique on deep learning models to produce uncertainty estimations for radiation therapy dose prediction. We show that both models are capable of generating a reasonable uncertainty map, and, with our proposed scaling technique, creating interpretable uncertainties and bounds on the prediction and any relevant metrics. Performance-wise, bagging provides statistically significant reduced loss value and errors in most of the metrics investigated in this study. The addition of bagging was able to further reduce errors by another 0.34% for Dmean and 0.19% for Dmax, on average, when compared to the baseline framework. Overall, the bagging framework provided significantly lower MAE of 2.62, as opposed to the baseline framework's MAE of 2.87. The usefulness of bagging, from solely a performance standpoint, does highly depend on the problem and the acceptable predictive error, and its high upfront computational cost during training should be factored in to deciding whether it is advantageous to use it. In terms of deployment with uncertainty estimations turned on, both frameworks offer the same performance time of about 12 seconds. As an ensemble-based metaheuristic, bagging can be used with existing machine learning architectures to improve stability and performance, and MCDO can be applied to any deep learning models that have dropout as part of their architecture.
Using Deep Learning to Predict Beam-Tunable Pareto Optimal Dose Distribution for Intensity Modulated Radiation Therapy
Jun 19, 2020

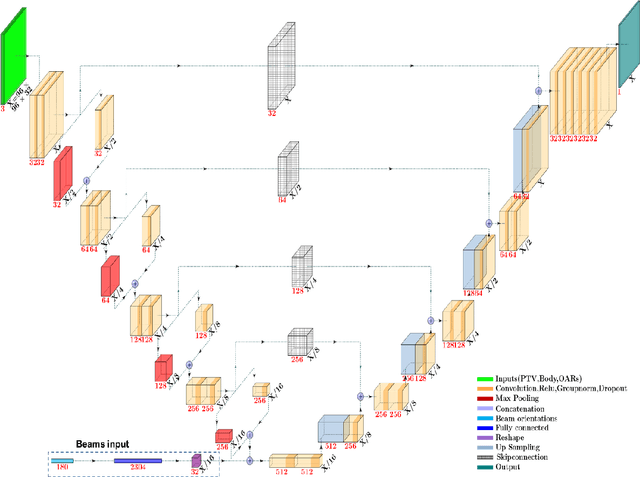
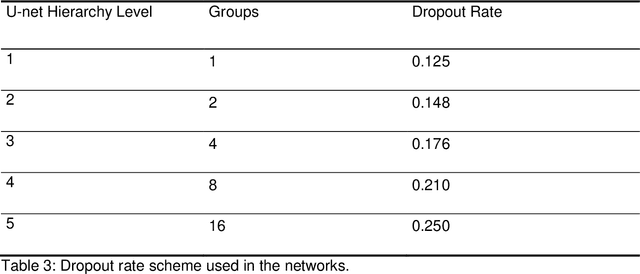
Abstract:We propose to develop deep learning models that can predict Pareto optimal dose distributions by using any given set of beam angles, along with patient anatomy, as input to train the deep neural networks. We implement and compare two deep learning networks that predict with two different beam configuration modalities. We generated Pareto optimal plans for 70 patients with prostate cancer. We used fluence map optimization to generate 500 IMRT plans that sampled the Pareto surface for each patient, for a total of 35,000 plans. We studied and compared two different models, Model I and Model II. Model I directly uses beam angles as a second input to the network as a binary vector. Model II converts the beam angles into beam doses that are conformal to the PTV. Our deep learning models predicted voxel-level dose distributions that precisely matched the ground truth dose distributions. Quantitatively, Model I prediction error of 0.043 (confirmation), 0.043 (homogeneity), 0.327 (R50), 2.80% (D95), 3.90% (D98), 0.6% (D50), 1.10% (D2) was lower than that of Model II, which obtained 0.076 (confirmation), 0.058 (homogeneity), 0.626 (R50), 7.10% (D95), 6.50% (D98), 8.40% (D50), 6.30% (D2). Treatment planners who use our models will be able to use deep learning to control the tradeoffs between the PTV and OAR weights, as well as the beam number and configurations in real time. Our dose prediction methods provide a stepping stone to building automatic IMRT treatment planning.
A reinforcement learning application of guided Monte Carlo Tree Search algorithm for beam orientation selection in radiation therapy
Apr 14, 2020



Abstract:Due to the large combinatorial problem, current beam orientation optimization algorithms for radiotherapy, such as column generation (CG), are typically heuristic or greedy in nature, leading to suboptimal solutions. We propose a reinforcement learning strategy using Monte Carlo Tree Search capable of finding a superior beam orientation set and in less time than CG.We utilized a reinforcement learning structure involving a supervised learning network to guide Monte Carlo tree search (GTS) to explore the decision space of beam orientation selection problem. We have previously trained a deep neural network (DNN) that takes in the patient anatomy, organ weights, and current beams, and then approximates beam fitness values, indicating the next best beam to add. This DNN is used to probabilistically guide the traversal of the branches of the Monte Carlo decision tree to add a new beam to the plan. To test the feasibility of the algorithm, we solved for 5-beam plans, using 13 test prostate cancer patients, different from the 57 training and validation patients originally trained the DNN. To show the strength of GTS to other search methods, performances of three other search methods including a guided search, uniform tree search and random search algorithms are also provided. On average GTS outperforms all other methods, it find a solution better than CG in 237 seconds on average, compared to CG which takes 360 seconds, and outperforms all other methods in finding a solution with lower objective function value in less than 1000 seconds. Using our guided tree search (GTS) method we were able to maintain a similar planning target volume (PTV) coverage within 1% error, and reduce the organ at risk (OAR) mean dose for body, rectum, left and right femoral heads, but a slight increase of 1% in bladder mean dose.
Incorporating human and learned domain knowledge into training deep neural networks: A differentiable dose volume histogram and adversarial inspired framework for generating Pareto optimal dose distributions in radiation therapy
Aug 16, 2019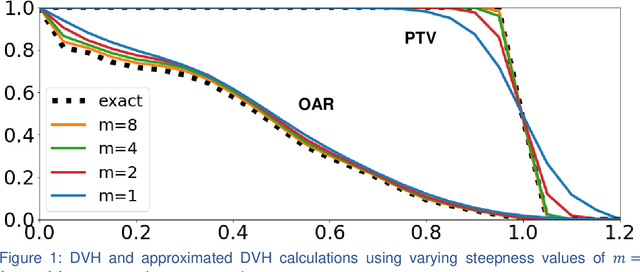
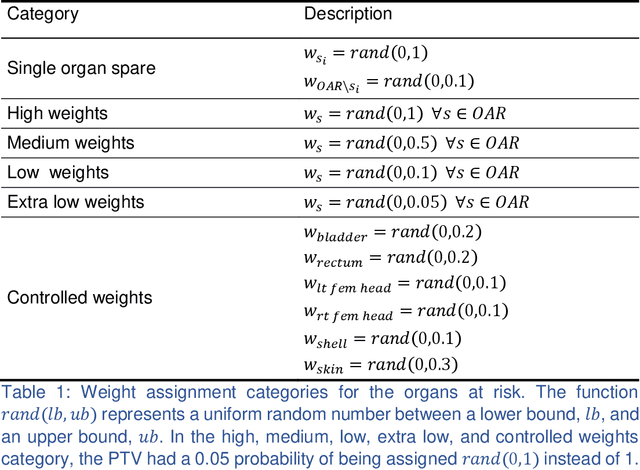
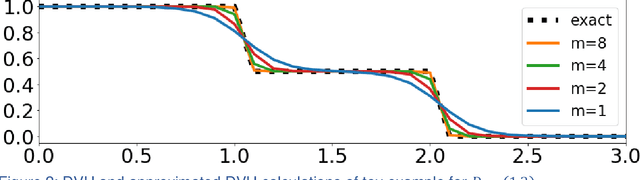

Abstract:We propose a novel domain specific loss, which is a differentiable loss function based on the dose volume histogram, and combine it with an adversarial loss for the training of deep neural networks to generate Pareto optimal dose distributions. The mean squared error (MSE) loss, dose volume histogram (DVH) loss, and adversarial (ADV) loss were used to train 4 instances of the neural network model: 1) MSE, 2) MSE+ADV, 3) MSE+DVH, and 4) MSE+DVH+ADV. 70 prostate patients were acquired, and the dose influence arrays were calculated for each patient. 1200 Pareto surface plans per patient were generated by pseudo-randomizing the tradeoff weights (84,000 plans total). We divided the data into 54 training, 6 validation, and 10 testing patients. Each model was trained for 100,000 iterations, with a batch size of 2. The prediction time of each model is 0.052 seconds. Quantitatively, the MSE+DVH+ADV model had the lowest prediction error of 0.038 (conformation), 0.026 (homogeneity), 0.298 (R50), 1.65% (D95), 2.14% (D98), 2.43% (D99). The MSE model had the worst prediction error of 0.134 (conformation), 0.041 (homogeneity), 0.520 (R50), 3.91% (D95), 4.33% (D98), 4.60% (D99). For both the mean dose PTV error and the max dose PTV, Body, Bladder and rectum error, the MSE+DVH+ADV outperformed all other models. All model's predictions have an average mean and max dose error less than 2.8% and 4.2%, respectively. Expert human domain specific knowledge can be the largest driver in the performance improvement, and adversarial learning can be used to further capture nuanced features. The real-time prediction capabilities allow for a physician to quickly navigate the tradeoff space, and produce a dose distribution as a tangible endpoint for the dosimetrist to use for planning. This can considerably reduce the treatment planning time, allowing for clinicians to focus their efforts on challenging cases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge