Jiaxiang Liu
ERNIE 5.0 Technical Report
Feb 04, 2026Abstract:In this report, we introduce ERNIE 5.0, a natively autoregressive foundation model desinged for unified multimodal understanding and generation across text, image, video, and audio. All modalities are trained from scratch under a unified next-group-of-tokens prediction objective, based on an ultra-sparse mixture-of-experts (MoE) architecture with modality-agnostic expert routing. To address practical challenges in large-scale deployment under diverse resource constraints, ERNIE 5.0 adopts a novel elastic training paradigm. Within a single pre-training run, the model learns a family of sub-models with varying depths, expert capacities, and routing sparsity, enabling flexible trade-offs among performance, model size, and inference latency in memory- or time-constrained scenarios. Moreover, we systematically address the challenges of scaling reinforcement learning to unified foundation models, thereby guaranteeing efficient and stable post-training under ultra-sparse MoE architectures and diverse multimodal settings. Extensive experiments demonstrate that ERNIE 5.0 achieves strong and balanced performance across multiple modalities. To the best of our knowledge, among publicly disclosed models, ERNIE 5.0 represents the first production-scale realization of a trillion-parameter unified autoregressive model that supports both multimodal understanding and generation. To facilitate further research, we present detailed visualizations of modality-agnostic expert routing in the unified model, alongside comprehensive empirical analysis of elastic training, aiming to offer profound insights to the community.
BMAM: Brain-inspired Multi-Agent Memory Framework
Jan 28, 2026Abstract:Language-model-based agents operating over extended interaction horizons face persistent challenges in preserving temporally grounded information and maintaining behavioral consistency across sessions, a failure mode we term soul erosion. We present BMAM (Brain-inspired Multi-Agent Memory), a general-purpose memory architecture that models agent memory as a set of functionally specialized subsystems rather than a single unstructured store. Inspired by cognitive memory systems, BMAM decomposes memory into episodic, semantic, salience-aware, and control-oriented components that operate at complementary time scales. To support long-horizon reasoning, BMAM organizes episodic memories along explicit timelines and retrieves evidence by fusing multiple complementary signals. Experiments on the LoCoMo benchmark show that BMAM achieves 78.45 percent accuracy under the standard long-horizon evaluation setting, and ablation analyses confirm that the hippocampus-inspired episodic memory subsystem plays a critical role in temporal reasoning.
Self-Calibrated Consistency can Fight Back for Adversarial Robustness in Vision-Language Models
Oct 26, 2025Abstract:Pre-trained vision-language models (VLMs) such as CLIP have demonstrated strong zero-shot capabilities across diverse domains, yet remain highly vulnerable to adversarial perturbations that disrupt image-text alignment and compromise reliability. Existing defenses typically rely on adversarial fine-tuning with labeled data, limiting their applicability in zero-shot settings. In this work, we identify two key weaknesses of current CLIP adversarial attacks -- lack of semantic guidance and vulnerability to view variations -- collectively termed semantic and viewpoint fragility. To address these challenges, we propose Self-Calibrated Consistency (SCC), an effective test-time defense. SCC consists of two complementary modules: Semantic consistency, which leverages soft pseudo-labels from counterattack warm-up and multi-view predictions to regularize cross-modal alignment and separate the target embedding from confusable negatives; and Spatial consistency, aligning perturbed visual predictions via augmented views to stabilize inference under adversarial perturbations. Together, these modules form a plug-and-play inference strategy. Extensive experiments on 22 benchmarks under diverse attack settings show that SCC consistently improves the zero-shot robustness of CLIP while maintaining accuracy, and can be seamlessly integrated with other VLMs for further gains. These findings highlight the great potential of establishing an adversarially robust paradigm from CLIP, with implications extending to broader vision-language domains such as BioMedCLIP.
Modest-Align: Data-Efficient Alignment for Vision-Language Models
Oct 24, 2025Abstract:Cross-modal alignment aims to map heterogeneous modalities into a shared latent space, as exemplified by models like CLIP, which benefit from large-scale image-text pretraining for strong recognition capabilities. However, when operating in resource-constrained settings with limited or low-quality data, these models often suffer from overconfidence and degraded performance due to the prevalence of ambiguous or weakly correlated image-text pairs. Current contrastive learning approaches, which rely on single positive pairs, further exacerbate this issue by reinforcing overconfidence on uncertain samples. To address these challenges, we propose Modest-Align, a lightweight alignment framework designed for robustness and efficiency. Our approach leverages two complementary strategies -- Random Perturbation, which introduces controlled noise to simulate uncertainty, and Embedding Smoothing, which calibrates similarity distributions in the embedding space. These mechanisms collectively reduce overconfidence and improve performance on noisy or weakly aligned samples. Extensive experiments across multiple benchmark datasets demonstrate that Modest-Align outperforms state-of-the-art methods in retrieval tasks, achieving competitive results with over 100x less training data and 600x less GPU time than CLIP. Our method offers a practical and scalable solution for cross-modal alignment in real-world, low-resource scenarios.
An approach for systematic decomposition of complex llm tasks
Oct 09, 2025Abstract:Large Language Models (LLMs) suffer from reliability issues on complex tasks, as existing decomposition methods are heuristic and rely on agent or manual decomposition. This work introduces a novel, systematic decomposition framework that we call Analysis of CONstraint-Induced Complexity (ACONIC), which models the task as a constraint problem and leveraging formal complexity measures to guide decomposition. On combinatorial (SATBench) and LLM database querying tasks (Spider), we find that by decomposing the tasks following the measure of complexity, agent can perform considerably better (10-40 percentage point).
3D-RAD: A Comprehensive 3D Radiology Med-VQA Dataset with Multi-Temporal Analysis and Diverse Diagnostic Tasks
Jun 11, 2025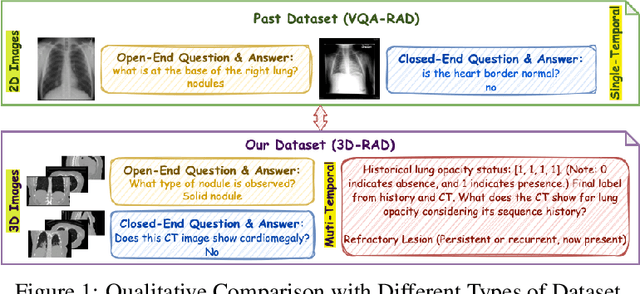
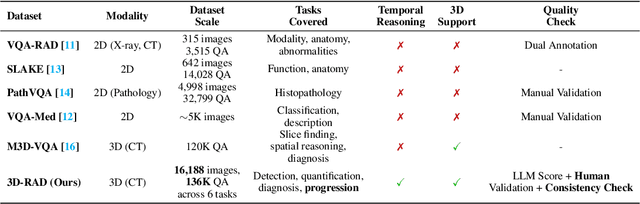
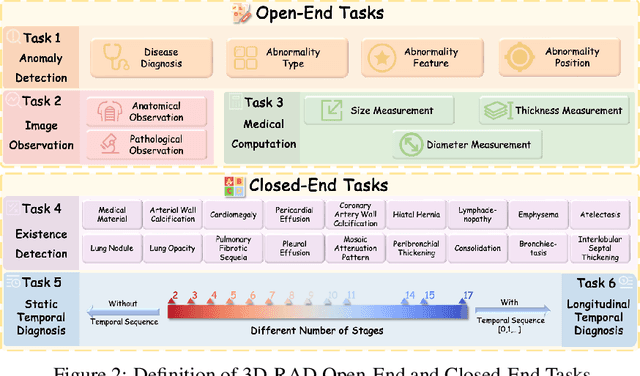
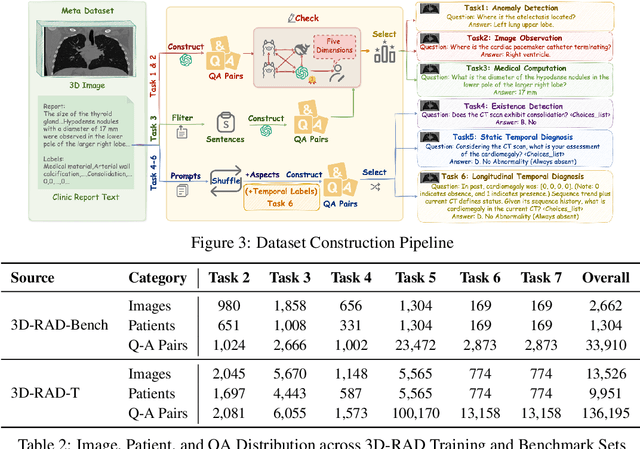
Abstract:Medical Visual Question Answering (Med-VQA) holds significant potential for clinical decision support, yet existing efforts primarily focus on 2D imaging with limited task diversity. This paper presents 3D-RAD, a large-scale dataset designed to advance 3D Med-VQA using radiology CT scans. The 3D-RAD dataset encompasses six diverse VQA tasks: anomaly detection, image observation, medical computation, existence detection, static temporal diagnosis, and longitudinal temporal diagnosis. It supports both open- and closed-ended questions while introducing complex reasoning challenges, including computational tasks and multi-stage temporal analysis, to enable comprehensive benchmarking. Extensive evaluations demonstrate that existing vision-language models (VLMs), especially medical VLMs exhibit limited generalization, particularly in multi-temporal tasks, underscoring the challenges of real-world 3D diagnostic reasoning. To drive future advancements, we release a high-quality training set 3D-RAD-T of 136,195 expert-aligned samples, showing that fine-tuning on this dataset could significantly enhance model performance. Our dataset and code, aiming to catalyze multimodal medical AI research and establish a robust foundation for 3D medical visual understanding, are publicly available at https://github.com/Tang-xiaoxiao/M3D-RAD.
Know-MRI: A Knowledge Mechanisms Revealer&Interpreter for Large Language Models
Jun 10, 2025Abstract:As large language models (LLMs) continue to advance, there is a growing urgency to enhance the interpretability of their internal knowledge mechanisms. Consequently, many interpretation methods have emerged, aiming to unravel the knowledge mechanisms of LLMs from various perspectives. However, current interpretation methods differ in input data formats and interpreting outputs. The tools integrating these methods are only capable of supporting tasks with specific inputs, significantly constraining their practical applications. To address these challenges, we present an open-source Knowledge Mechanisms Revealer&Interpreter (Know-MRI) designed to analyze the knowledge mechanisms within LLMs systematically. Specifically, we have developed an extensible core module that can automatically match different input data with interpretation methods and consolidate the interpreting outputs. It enables users to freely choose appropriate interpretation methods based on the inputs, making it easier to comprehensively diagnose the model's internal knowledge mechanisms from multiple perspectives. Our code is available at https://github.com/nlpkeg/Know-MRI. We also provide a demonstration video on https://youtu.be/NVWZABJ43Bs.
Leveraging Pretrained Diffusion Models for Zero-Shot Part Assembly
May 01, 2025Abstract:3D part assembly aims to understand part relationships and predict their 6-DoF poses to construct realistic 3D shapes, addressing the growing demand for autonomous assembly, which is crucial for robots. Existing methods mainly estimate the transformation of each part by training neural networks under supervision, which requires a substantial quantity of manually labeled data. However, the high cost of data collection and the immense variability of real-world shapes and parts make traditional methods impractical for large-scale applications. In this paper, we propose first a zero-shot part assembly method that utilizes pre-trained point cloud diffusion models as discriminators in the assembly process, guiding the manipulation of parts to form realistic shapes. Specifically, we theoretically demonstrate that utilizing a diffusion model for zero-shot part assembly can be transformed into an Iterative Closest Point (ICP) process. Then, we propose a novel pushing-away strategy to address the overlap parts, thereby further enhancing the robustness of the method. To verify our work, we conduct extensive experiments and quantitative comparisons to several strong baseline methods, demonstrating the effectiveness of the proposed approach, which even surpasses the supervised learning method. The code has been released on https://github.com/Ruiyuan-Zhang/Zero-Shot-Assembly.
* 10 pages, 12 figures, Accepted by IJCAI-2025
Capability Localization: Capabilities Can be Localized rather than Individual Knowledge
Feb 28, 2025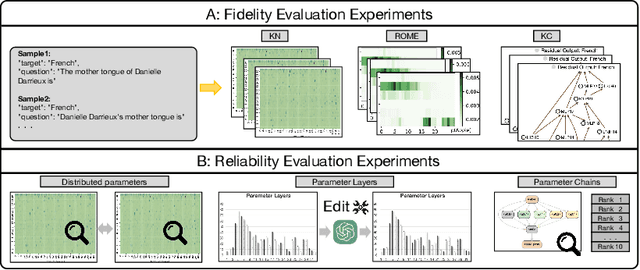
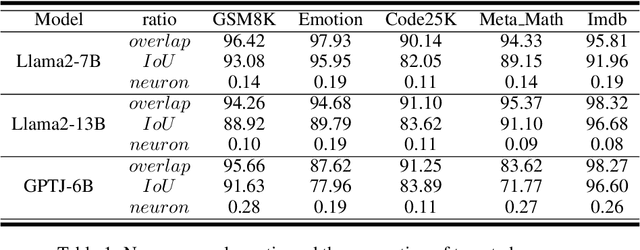
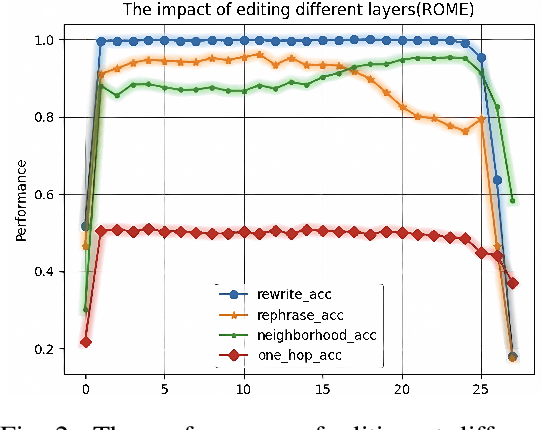
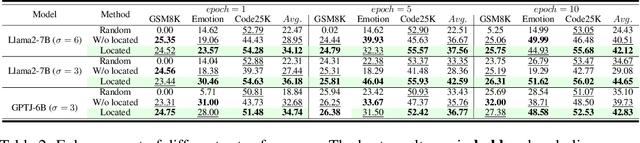
Abstract:Large scale language models have achieved superior performance in tasks related to natural language processing, however, it is still unclear how model parameters affect performance improvement. Previous studies assumed that individual knowledge is stored in local parameters, and the storage form of individual knowledge is dispersed parameters, parameter layers, or parameter chains, which are not unified. We found through fidelity and reliability evaluation experiments that individual knowledge cannot be localized. Afterwards, we constructed a dataset for decoupling experiments and discovered the potential for localizing data commonalities. To further reveal this phenomenon, this paper proposes a Commonality Neuron Localization (CNL) method, which successfully locates commonality neurons and achieves a neuron overlap rate of 96.42% on the GSM8K dataset. Finally, we have demonstrated through cross data experiments that commonality neurons are a collection of capability neurons that possess the capability to enhance performance. Our code is available at https://github.com/nlpkeg/Capability-Neuron-Localization.
Fair-MoE: Fairness-Oriented Mixture of Experts in Vision-Language Models
Feb 10, 2025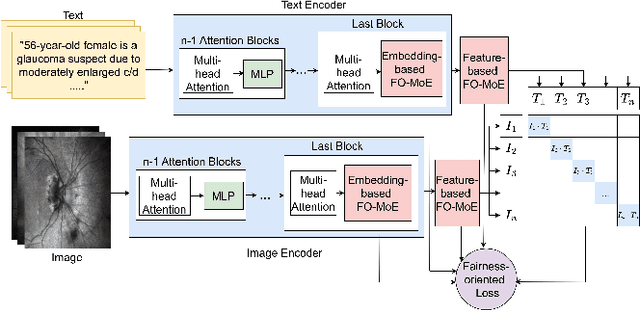
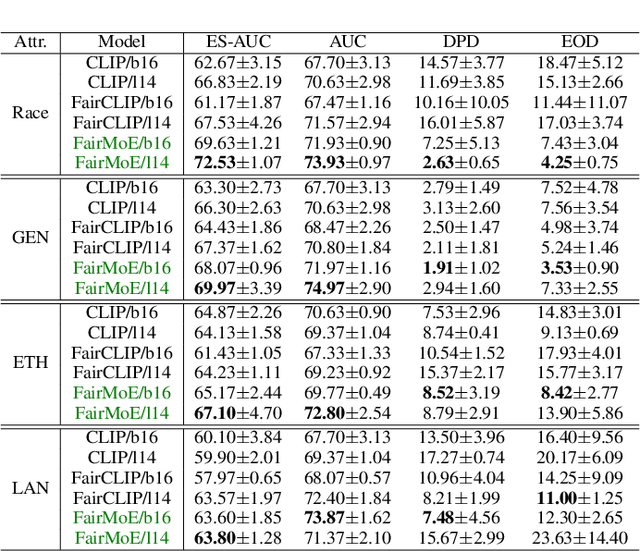
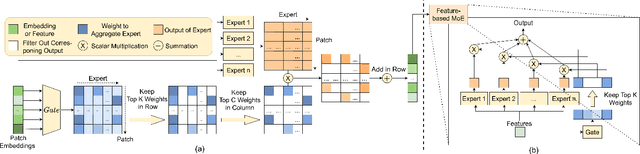
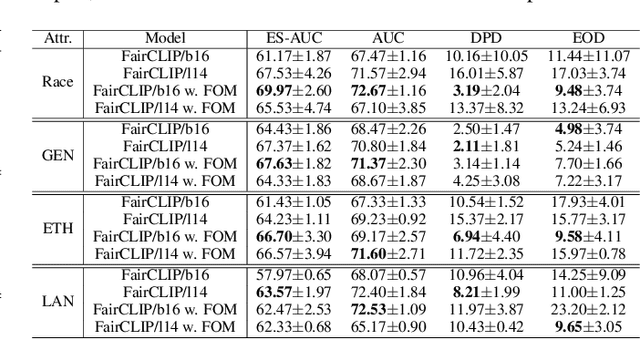
Abstract:Fairness is a fundamental principle in medical ethics. Vision Language Models (VLMs) have shown significant potential in the medical field due to their ability to leverage both visual and linguistic contexts, reducing the need for large datasets and enabling the performance of complex tasks. However, the exploration of fairness within VLM applications remains limited. Applying VLMs without a comprehensive analysis of fairness could lead to concerns about equal treatment opportunities and diminish public trust in medical deep learning models. To build trust in medical VLMs, we propose Fair-MoE, a model specifically designed to ensure both fairness and effectiveness. Fair-MoE comprises two key components: \textit{the Fairness-Oriented Mixture of Experts (FO-MoE)} and \textit{the Fairness-Oriented Loss (FOL)}. FO-MoE is designed to leverage the expertise of various specialists to filter out biased patch embeddings and use an ensemble approach to extract more equitable information relevant to specific tasks. FOL is a novel fairness-oriented loss function that not only minimizes the distances between different attributes but also optimizes the differences in the dispersion of various attributes' distributions. Extended experiments demonstrate the effectiveness and fairness of Fair-MoE. Tested on the Harvard-FairVLMed dataset, Fair-MoE showed improvements in both fairness and accuracy across all four attributes. Code will be publicly available.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge