Sanjay Talbar
Generative Adversarial Networks based Skin Lesion Segmentation
May 29, 2023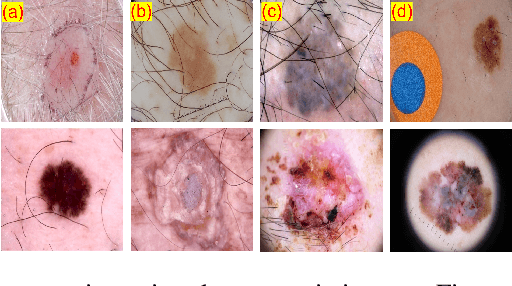
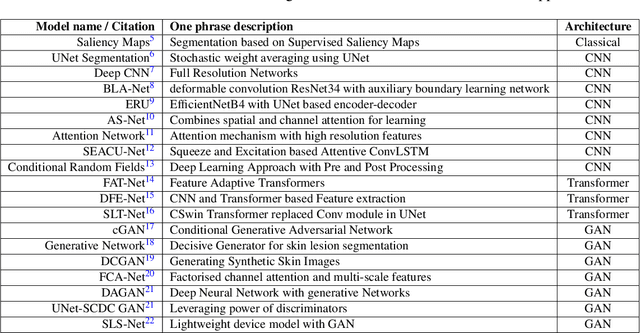
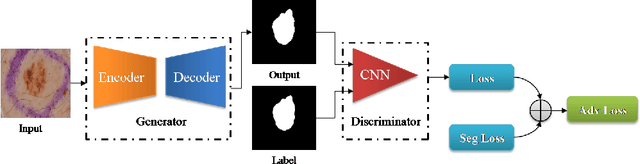
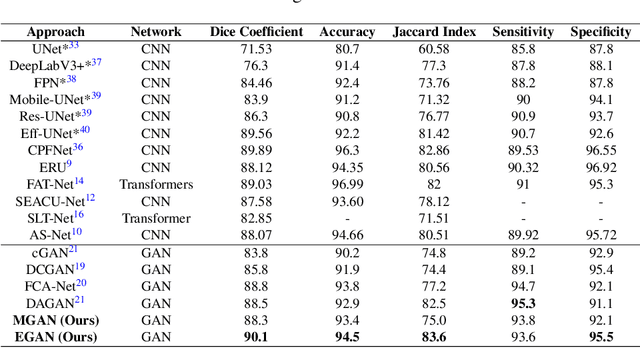
Abstract:Skin cancer is a serious condition that requires accurate identification and treatment. One way to assist clinicians in this task is by using computer-aided diagnosis (CAD) tools that can automatically segment skin lesions from dermoscopic images. To this end, a new adversarial learning-based framework called EGAN has been developed. This framework uses an unsupervised generative network to generate accurate lesion masks. It consists of a generator module with a top-down squeeze excitation-based compound scaled path and an asymmetric lateral connection-based bottom-up path, and a discriminator module that distinguishes between original and synthetic masks. Additionally, a morphology-based smoothing loss is implemented to encourage the network to create smooth semantic boundaries of lesions. The framework is evaluated on the International Skin Imaging Collaboration (ISIC) Lesion Dataset 2018 and outperforms the current state-of-the-art skin lesion segmentation approaches with a Dice coefficient, Jaccard similarity, and Accuracy of 90.1%, 83.6%, and 94.5%, respectively. This represents a 2% increase in Dice Coefficient, 1% increase in Jaccard Index, and 1% increase in Accuracy.
Deep Learning based Novel Cascaded Approach for Skin Lesion Analysis
Jan 16, 2023



Abstract:Automatic lesion analysis is critical in skin cancer diagnosis and ensures effective treatment. The computer aided diagnosis of such skin cancer in dermoscopic images can significantly reduce the clinicians workload and help improve diagnostic accuracy. Although researchers are working extensively to address this problem, early detection and accurate identification of skin lesions remain challenging. This research focuses on a two step framework for skin lesion segmentation followed by classification for lesion analysis. We explored the effectiveness of deep convolutional neural network based architectures by designing an encoder-decoder architecture for skin lesion segmentation and CNN based classification network. The proposed approaches are evaluated quantitatively in terms of the Accuracy, mean Intersection over Union and Dice Similarity Coefficient. Our cascaded end to end deep learning based approach is the first of its kind, where the classification accuracy of the lesion is significantly improved because of prior segmentation.
QU-BraTS: MICCAI BraTS 2020 Challenge on Quantifying Uncertainty in Brain Tumor Segmentation -- Analysis of Ranking Metrics and Benchmarking Results
Dec 19, 2021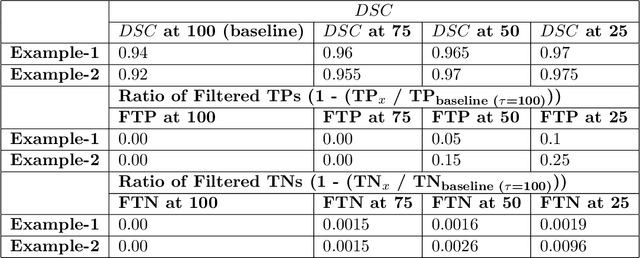
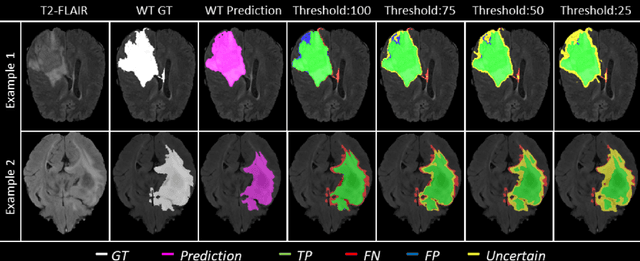
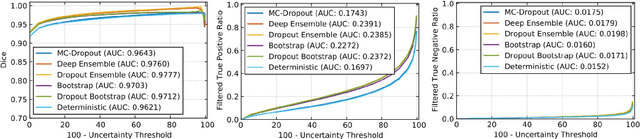
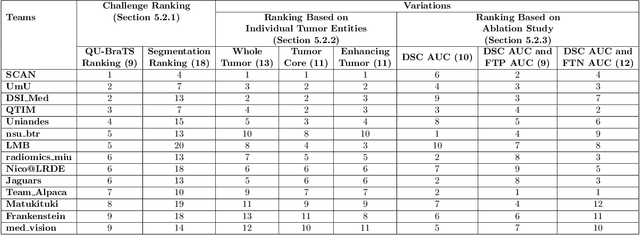
Abstract:Deep learning (DL) models have provided the state-of-the-art performance in a wide variety of medical imaging benchmarking challenges, including the Brain Tumor Segmentation (BraTS) challenges. However, the task of focal pathology multi-compartment segmentation (e.g., tumor and lesion sub-regions) is particularly challenging, and potential errors hinder the translation of DL models into clinical workflows. Quantifying the reliability of DL model predictions in the form of uncertainties, could enable clinical review of the most uncertain regions, thereby building trust and paving the way towards clinical translation. Recently, a number of uncertainty estimation methods have been introduced for DL medical image segmentation tasks. Developing metrics to evaluate and compare the performance of uncertainty measures will assist the end-user in making more informed decisions. In this study, we explore and evaluate a metric developed during the BraTS 2019-2020 task on uncertainty quantification (QU-BraTS), and designed to assess and rank uncertainty estimates for brain tumor multi-compartment segmentation. This metric (1) rewards uncertainty estimates that produce high confidence in correct assertions, and those that assign low confidence levels at incorrect assertions, and (2) penalizes uncertainty measures that lead to a higher percentages of under-confident correct assertions. We further benchmark the segmentation uncertainties generated by 14 independent participating teams of QU-BraTS 2020, all of which also participated in the main BraTS segmentation task. Overall, our findings confirm the importance and complementary value that uncertainty estimates provide to segmentation algorithms, and hence highlight the need for uncertainty quantification in medical image analyses. Our evaluation code is made publicly available at https://github.com/RagMeh11/QU-BraTS.
Colorectal Cancer Segmentation using Atrous Convolution and Residual Enhanced UNet
Mar 16, 2021



Abstract:Colorectal cancer is a leading cause of death worldwide. However, early diagnosis dramatically increases the chances of survival, for which it is crucial to identify the tumor in the body. Since its imaging uses high-resolution techniques, annotating the tumor is time-consuming and requires particular expertise. Lately, methods built upon Convolutional Neural Networks(CNNs) have proven to be at par, if not better in many biomedical segmentation tasks. For the task at hand, we propose another CNN-based approach, which uses atrous convolutions and residual connections besides the conventional filters. The training and inference were made using an efficient patch-based approach, which significantly reduced unnecessary computations. The proposed AtResUNet was trained on the DigestPath 2019 Challenge dataset for colorectal cancer segmentation with results having a Dice Coefficient of 0.748.
The 1st Agriculture-Vision Challenge: Methods and Results
Apr 23, 2020
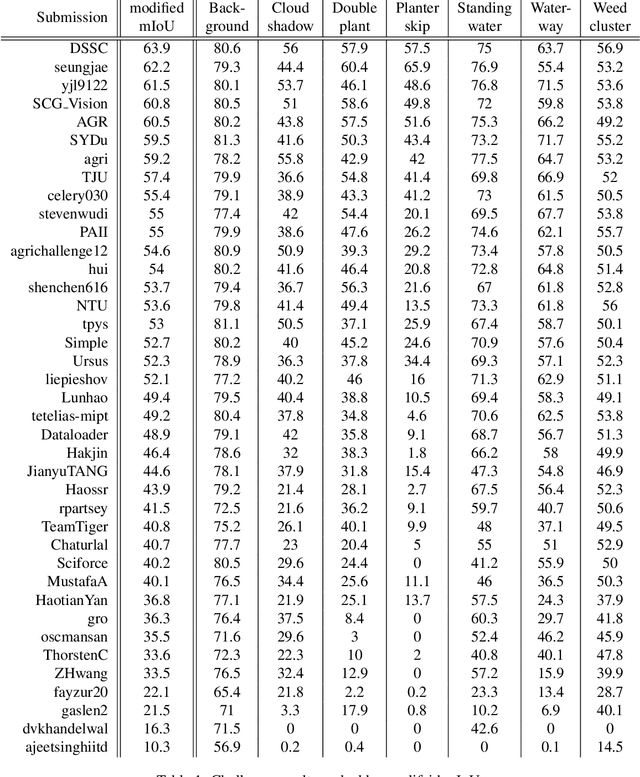
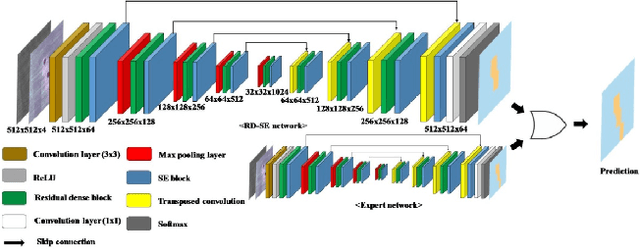
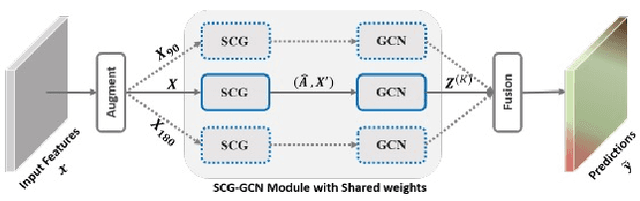
Abstract:The first Agriculture-Vision Challenge aims to encourage research in developing novel and effective algorithms for agricultural pattern recognition from aerial images, especially for the semantic segmentation task associated with our challenge dataset. Around 57 participating teams from various countries compete to achieve state-of-the-art in aerial agriculture semantic segmentation. The Agriculture-Vision Challenge Dataset was employed, which comprises of 21,061 aerial and multi-spectral farmland images. This paper provides a summary of notable methods and results in the challenge. Our submission server and leaderboard will continue to open for researchers that are interested in this challenge dataset and task; the link can be found here.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge