Pamela Ventola
STNAGNN: Spatiotemporal Node Attention Graph Neural Network for Task-based fMRI Analysis
Jun 17, 2024Abstract:Task-based fMRI uses actions or stimuli to trigger task-specific brain responses and measures them using BOLD contrast. Despite the significant task-induced spatiotemporal brain activation fluctuations, most studies on task-based fMRI ignore the task context information aligned with fMRI and consider task-based fMRI a coherent sequence. In this paper, we show that using the task structures as data-driven guidance is effective for spatiotemporal analysis. We propose STNAGNN, a GNN-based spatiotemporal architecture, and validate its performance in an autism classification task. The trained model is also interpreted for identifying autism-related spatiotemporal brain biomarkers.
Learning Oculomotor Behaviors from Scanpath
Aug 11, 2021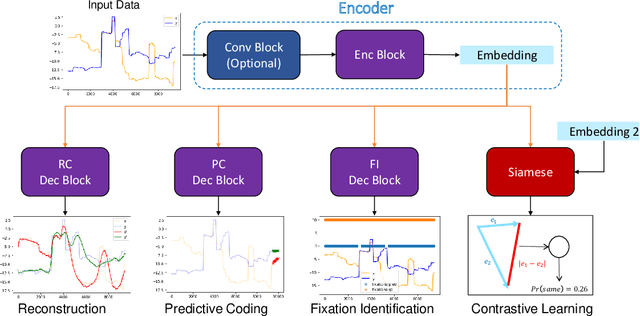
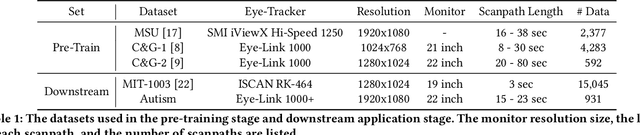
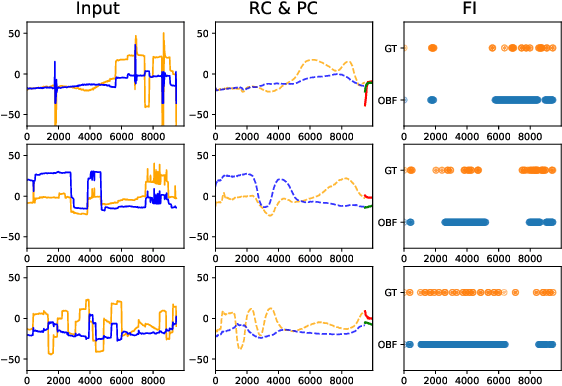
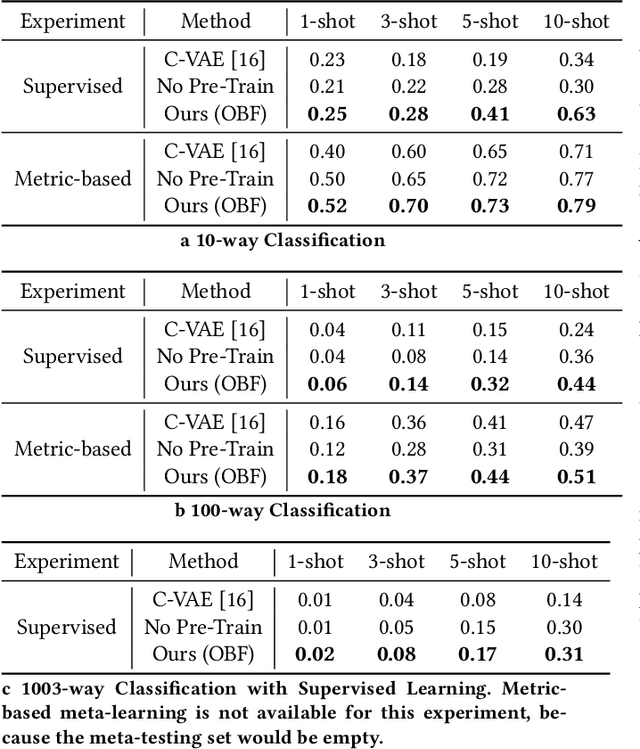
Abstract:Identifying oculomotor behaviors relevant for eye-tracking applications is a critical but often challenging task. Aiming to automatically learn and extract knowledge from existing eye-tracking data, we develop a novel method that creates rich representations of oculomotor scanpaths to facilitate the learning of downstream tasks. The proposed stimulus-agnostic Oculomotor Behavior Framework (OBF) model learns human oculomotor behaviors from unsupervised and semi-supervised tasks, including reconstruction, predictive coding, fixation identification, and contrastive learning tasks. The resultant pre-trained OBF model can be used in a variety of applications. Our pre-trained model outperforms baseline approaches and traditional scanpath methods in autism spectrum disorder and viewed-stimulus classification tasks. Ablation experiments further show our proposed method could achieve even better results with larger model sizes and more diverse eye-tracking training datasets, supporting the model's potential for future eye-tracking applications. Open source code: http://github.com/BeibinLi/OBF.
Estimating Reproducible Functional Networks Associated with Task Dynamics using Unsupervised LSTMs
May 06, 2021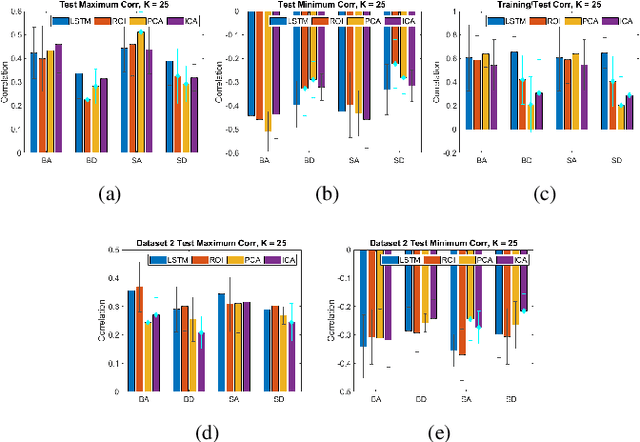
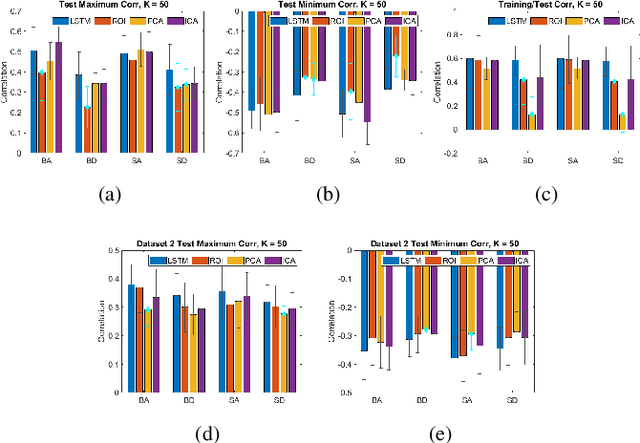
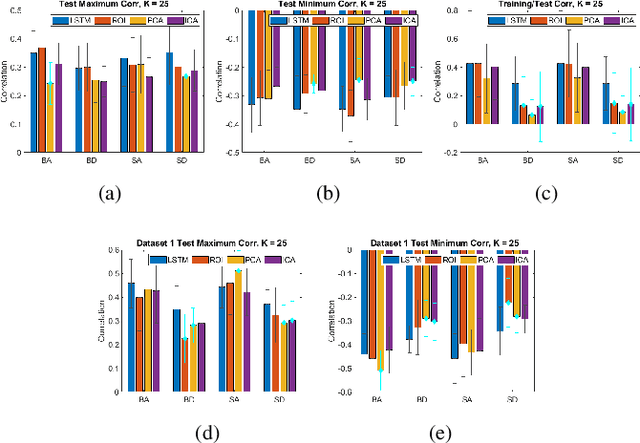
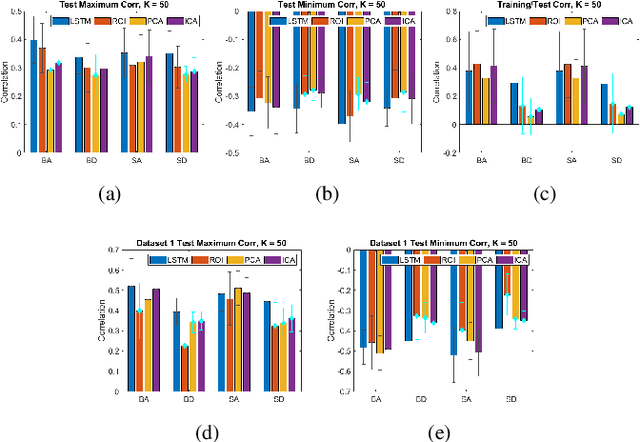
Abstract:We propose a method for estimating more reproducible functional networks that are more strongly associated with dynamic task activity by using recurrent neural networks with long short term memory (LSTMs). The LSTM model is trained in an unsupervised manner to learn to generate the functional magnetic resonance imaging (fMRI) time-series data in regions of interest. The learned functional networks can then be used for further analysis, e.g., correlation analysis to determine functional networks that are strongly associated with an fMRI task paradigm. We test our approach and compare to other methods for decomposing functional networks from fMRI activity on 2 related but separate datasets that employ a biological motion perception task. We demonstrate that the functional networks learned by the LSTM model are more strongly associated with the task activity and dynamics compared to other approaches. Furthermore, the patterns of network association are more closely replicated across subjects within the same dataset as well as across datasets. More reproducible functional networks are essential for better characterizing the neural correlates of a target task.
* IEEE International Symposium on Biomedical Imaging (ISBI) 2020
Demographic-Guided Attention in Recurrent Neural Networks for Modeling Neuropathophysiological Heterogeneity
Apr 15, 2021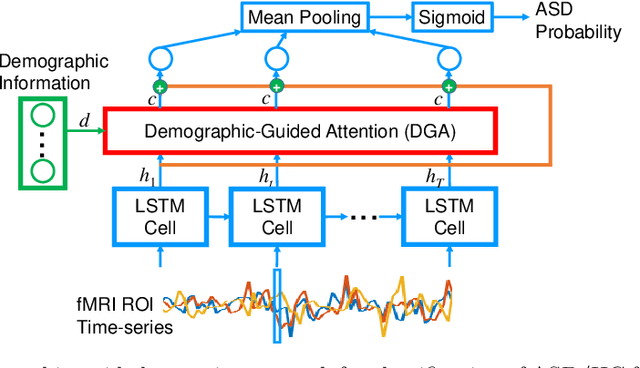
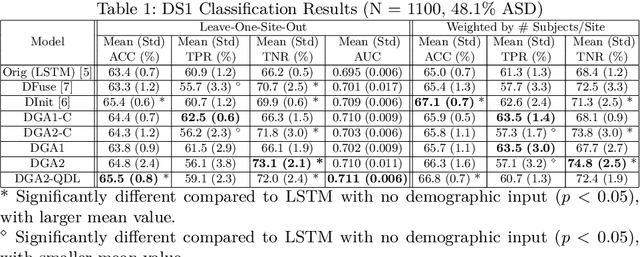
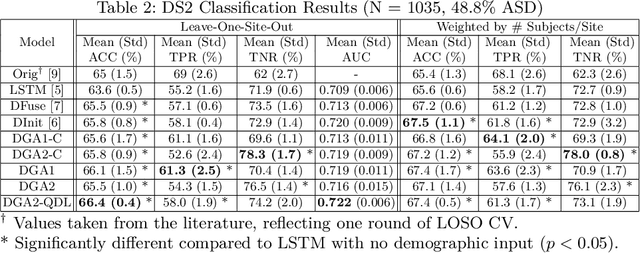
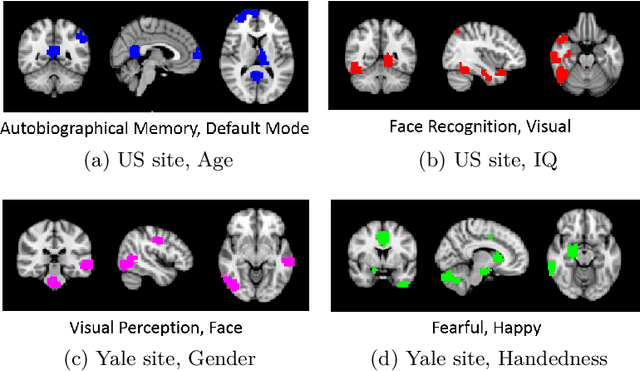
Abstract:Heterogeneous presentation of a neurological disorder suggests potential differences in the underlying pathophysiological changes that occur in the brain. We propose to model heterogeneous patterns of functional network differences using a demographic-guided attention (DGA) mechanism for recurrent neural network models for prediction from functional magnetic resonance imaging (fMRI) time-series data. The context computed from the DGA head is used to help focus on the appropriate functional networks based on individual demographic information. We demonstrate improved classification on 3 subsets of the ABIDE I dataset used in published studies that have previously produced state-of-the-art results, evaluating performance under a leave-one-site-out cross-validation framework for better generalizeability to new data. Finally, we provide examples of interpreting functional network differences based on individual demographic variables.
Multiple-shooting adjoint method for whole-brain dynamic causal modeling
Feb 14, 2021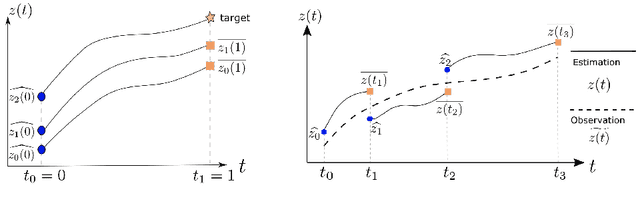

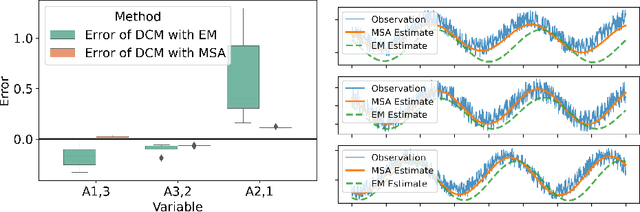
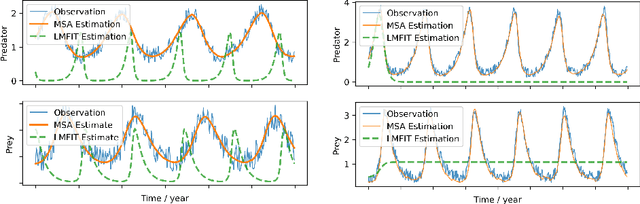
Abstract:Dynamic causal modeling (DCM) is a Bayesian framework to infer directed connections between compartments, and has been used to describe the interactions between underlying neural populations based on functional neuroimaging data. DCM is typically analyzed with the expectation-maximization (EM) algorithm. However, because the inversion of a large-scale continuous system is difficult when noisy observations are present, DCM by EM is typically limited to a small number of compartments ($<10$). Another drawback with the current method is its complexity; when the forward model changes, the posterior mean changes, and we need to re-derive the algorithm for optimization. In this project, we propose the Multiple-Shooting Adjoint (MSA) method to address these limitations. MSA uses the multiple-shooting method for parameter estimation in ordinary differential equations (ODEs) under noisy observations, and is suitable for large-scale systems such as whole-brain analysis in functional MRI (fMRI). Furthermore, MSA uses the adjoint method for accurate gradient estimation in the ODE; since the adjoint method is generic, MSA is a generic method for both linear and non-linear systems, and does not require re-derivation of the algorithm as in EM. We validate MSA in extensive experiments: 1) in toy examples with both linear and non-linear models, we show that MSA achieves better accuracy in parameter value estimation than EM; furthermore, MSA can be successfully applied to large systems with up to 100 compartments; and 2) using real fMRI data, we apply MSA to the estimation of the whole-brain effective connectome and show improved classification of autism spectrum disorder (ASD) vs. control compared to using the functional connectome. The package is provided \url{https://jzkay12.github.io/TorchDiffEqPack}
Pooling Regularized Graph Neural Network for fMRI Biomarker Analysis
Jul 29, 2020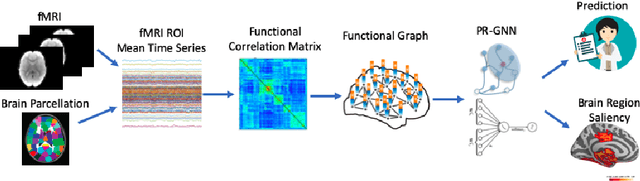
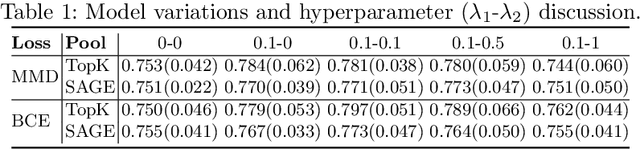
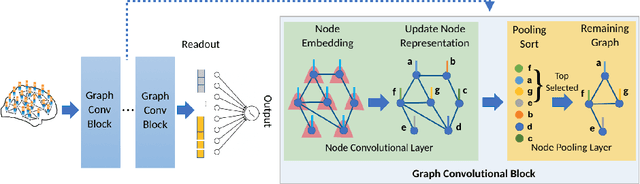
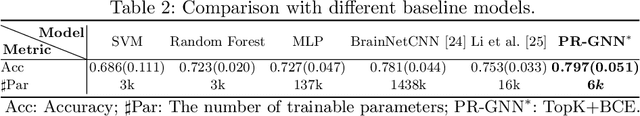
Abstract:Understanding how certain brain regions relate to a specific neurological disorder has been an important area of neuroimaging research. A promising approach to identify the salient regions is using Graph Neural Networks (GNNs), which can be used to analyze graph structured data, e.g. brain networks constructed by functional magnetic resonance imaging (fMRI). We propose an interpretable GNN framework with a novel salient region selection mechanism to determine neurological brain biomarkers associated with disorders. Specifically, we design novel regularized pooling layers that highlight salient regions of interests (ROIs) so that we can infer which ROIs are important to identify a certain disease based on the node pooling scores calculated by the pooling layers. Our proposed framework, Pooling Regularized-GNN (PR-GNN), encourages reasonable ROI-selection and provides flexibility to preserve either individual- or group-level patterns. We apply the PR-GNN framework on a Biopoint Autism Spectral Disorder (ASD) fMRI dataset. We investigate different choices of the hyperparameters and show that PR-GNN outperforms baseline methods in terms of classification accuracy. The salient ROI detection results show high correspondence with the previous neuroimaging-derived biomarkers for ASD.
* 11 pages, 4 figures
Multi-site fMRI Analysis Using Privacy-preserving Federated Learning and Domain Adaptation: ABIDE Results
Jan 16, 2020
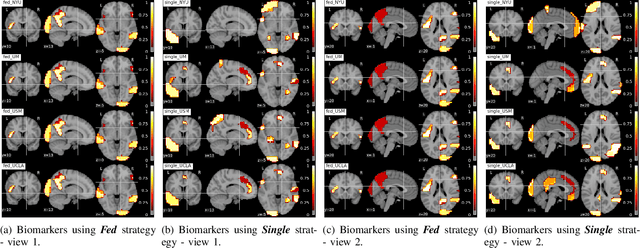
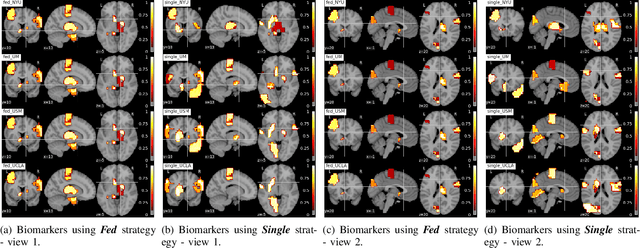
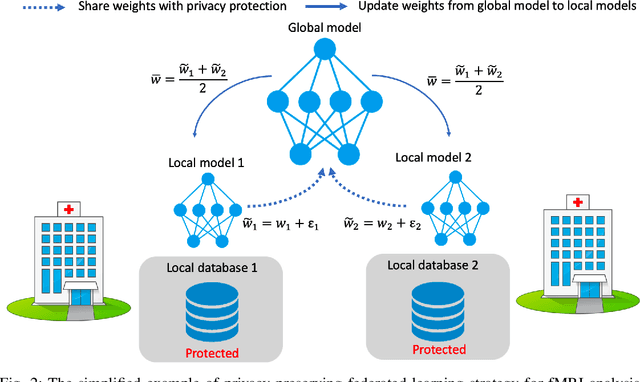
Abstract:Deep learning models have shown their advantage in many different tasks, including neuroimage analysis. However, to effectively train a high-quality deep learning model, the aggregation of a significant amount of patient information is required. The time and cost for acquisition and annotation in assembling, for example, large fMRI datasets make it difficult to acquire large numbers at a single site. However, due to the need to protect the privacy of patient data, it is hard to assemble a central database from multiple institutions. Federated learning allows for population-level models to be trained without centralizing entities' data by transmitting the global model to local entities, training the model locally, and then averaging the gradients or weights in the global model. However, some studies suggest that private information can be recovered from the model gradients or weights. In this work, we address the problem of multi-site fMRI classification with a privacy-preserving strategy. To solve the problem, we propose a federated learning approach, where a decentralized iterative optimization algorithm is implemented and shared local model weights are altered by a randomization mechanism. Considering the systemic differences of fMRI distributions from different sites, we further propose two domain adaptation methods in this federated learning formulation. We investigate various practical aspects of federated model optimization and compare federated learning with alternative training strategies. Overall, our results demonstrate that it is promising to utilize multi-site data without data sharing to boost neuroimage analysis performance and find reliable disease-related biomarkers. Our proposed pipeline can be generalized to other privacy-sensitive medical data analysis problems.
Sparsely Grouped Input Variables for Neural Networks
Nov 29, 2019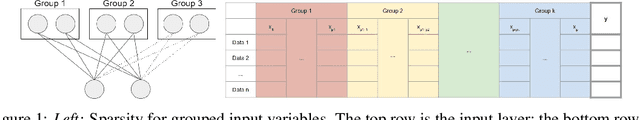

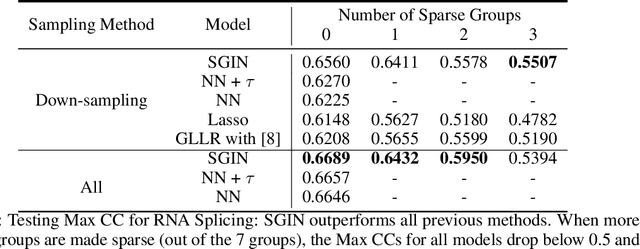
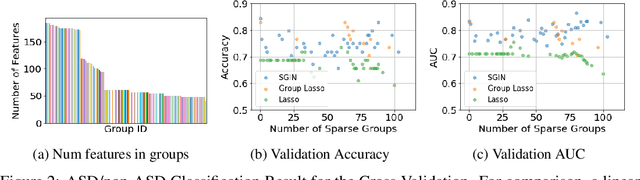
Abstract:In genomic analysis, biomarker discovery, image recognition, and other systems involving machine learning, input variables can often be organized into different groups by their source or semantic category. Eliminating some groups of variables can expedite the process of data acquisition and avoid over-fitting. Researchers have used the group lasso to ensure group sparsity in linear models and have extended it to create compact neural networks in meta-learning. Different from previous studies, we use multi-layer non-linear neural networks to find sparse groups for input variables. We propose a new loss function to regularize parameters for grouped input variables, design a new optimization algorithm for this loss function, and test these methods in three real-world settings. We achieve group sparsity for three datasets, maintaining satisfying results while excluding one nucleotide position from an RNA splicing experiment, excluding 89.9% of stimuli from an eye-tracking experiment, and excluding 60% of image rows from an experiment on the MNIST dataset.
Graph Embedding Using Infomax for ASD Classification and Brain Functional Difference Detection
Aug 14, 2019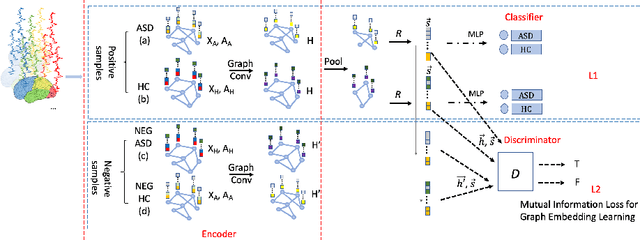

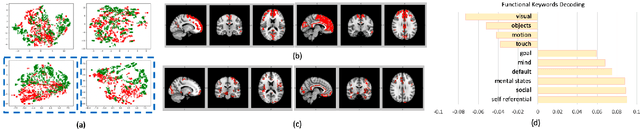
Abstract:Significant progress has been made using fMRI to characterize the brain changes that occur in ASD, a complex neuro-developmental disorder. However, due to the high dimensionality and low signal-to-noise ratio of fMRI, embedding informative and robust brain regional fMRI representations for both graph-level classification and region-level functional difference detection tasks between ASD and healthy control (HC) groups is difficult. Here, we model the whole brain fMRI as a graph, which preserves geometrical and temporal information and use a Graph Neural Network (GNN) to learn from the graph-structured fMRI data. We investigate the potential of including mutual information (MI) loss (Infomax), which is an unsupervised term encouraging large MI of each nodal representation and its corresponding graph-level summarized representation to learn a better graph embedding. Specifically, this work developed a pipeline including a GNN encoder, a classifier and a discriminator, which forces the encoded nodal representations to both benefit classification and reveal the common nodal patterns in a graph. We simultaneously optimize graph-level classification loss and Infomax. We demonstrated that Infomax graph embedding improves classification performance as a regularization term. Furthermore, we found separable nodal representations of ASD and HC groups in prefrontal cortex, cingulate cortex, visual regions, and other social, emotional and execution related brain regions. In contrast with GNN with classification loss only, the proposed pipeline can facilitate training more robust ASD classification models. Moreover, the separable nodal representations can detect the functional differences between the two groups and contribute to revealing new ASD biomarkers.
Invertible Network for Classification and Biomarker Selection for ASD
Jul 23, 2019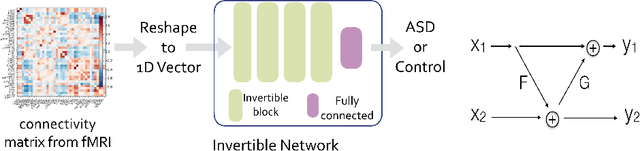
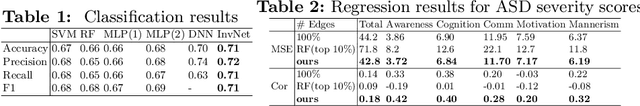
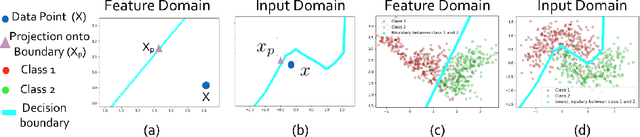
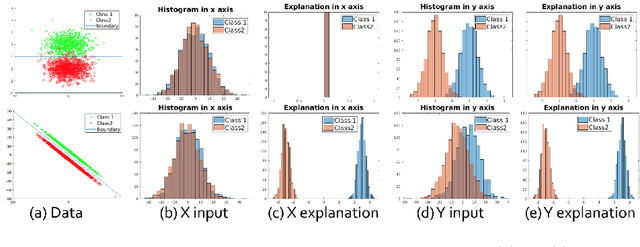
Abstract:Determining biomarkers for autism spectrum disorder (ASD) is crucial to understanding its mechanisms. Recently deep learning methods have achieved success in the classification task of ASD using fMRI data. However, due to the black-box nature of most deep learning models, it's hard to perform biomarker selection and interpret model decisions. The recently proposed invertible networks can accurately reconstruct the input from its output, and have the potential to unravel the black-box representation. Therefore, we propose a novel method to classify ASD and identify biomarkers for ASD using the connectivity matrix calculated from fMRI as the input. Specifically, with invertible networks, we explicitly determine the decision boundary and the projection of data points onto the boundary. Like linear classifiers, the difference between a point and its projection onto the decision boundary can be viewed as the explanation. We then define the importance as the explanation weighted by the gradient of prediction $w.r.t$ the input, and identify biomarkers based on this importance measure. We perform a regression task to further validate our biomarker selection: compared to using all edges in the connectivity matrix, using the top 10\% important edges we generate a lower regression error on 6 different severity scores. Our experiments show that the invertible network is both effective at ASD classification and interpretable, allowing for discovery of reliable biomarkers.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge