Nikos Paragios
Therapanacea
GuidedRec: Guiding Ill-Posed Unsupervised Volumetric Recovery
May 20, 2024



Abstract:We introduce a novel unsupervised approach to reconstructing a 3D volume from only two planar projections that exploits a previous\-ly-captured 3D volume of the patient. Such volume is readily available in many important medical procedures and previous methods already used such a volume. Earlier methods that work by deforming this volume to match the projections typically fail when the number of projections is very low as the alignment becomes underconstrained. We show how to use a generative model of the volume structures to constrain the deformation and obtain a correct estimate. Moreover, our method is not bounded to a specific sensor calibration and can be applied to new calibrations without retraining. We evaluate our approach on a challenging dataset and show it outperforms state-of-the-art methods. As a result, our method could be used in treatment scenarios such as surgery and radiotherapy while drastically reducing patient radiation exposure.
ToNNO: Tomographic Reconstruction of a Neural Network's Output for Weakly Supervised Segmentation of 3D Medical Images
Apr 19, 2024



Abstract:Annotating lots of 3D medical images for training segmentation models is time-consuming. The goal of weakly supervised semantic segmentation is to train segmentation models without using any ground truth segmentation masks. Our work addresses the case where only image-level categorical labels, indicating the presence or absence of a particular region of interest (such as tumours or lesions), are available. Most existing methods rely on class activation mapping (CAM). We propose a novel approach, ToNNO, which is based on the Tomographic reconstruction of a Neural Network's Output. Our technique extracts stacks of slices with different angles from the input 3D volume, feeds these slices to a 2D encoder, and applies the inverse Radon transform in order to reconstruct a 3D heatmap of the encoder's predictions. This generic method allows to perform dense prediction tasks on 3D volumes using any 2D image encoder. We apply it to weakly supervised medical image segmentation by training the 2D encoder to output high values for slices containing the regions of interest. We test it on four large scale medical image datasets and outperform 2D CAM methods. We then extend ToNNO by combining tomographic reconstruction with CAM methods, proposing Averaged CAM and Tomographic CAM, which obtain even better results.
Certification of Deep Learning Models for Medical Image Segmentation
Oct 05, 2023Abstract:In medical imaging, segmentation models have known a significant improvement in the past decade and are now used daily in clinical practice. However, similar to classification models, segmentation models are affected by adversarial attacks. In a safety-critical field like healthcare, certifying model predictions is of the utmost importance. Randomized smoothing has been introduced lately and provides a framework to certify models and obtain theoretical guarantees. In this paper, we present for the first time a certified segmentation baseline for medical imaging based on randomized smoothing and diffusion models. Our results show that leveraging the power of denoising diffusion probabilistic models helps us overcome the limits of randomized smoothing. We conduct extensive experiments on five public datasets of chest X-rays, skin lesions, and colonoscopies, and empirically show that we are able to maintain high certified Dice scores even for highly perturbed images. Our work represents the first attempt to certify medical image segmentation models, and we aspire for it to set a foundation for future benchmarks in this crucial and largely uncharted area.
The STOIC2021 COVID-19 AI challenge: applying reusable training methodologies to private data
Jun 25, 2023Abstract:Challenges drive the state-of-the-art of automated medical image analysis. The quantity of public training data that they provide can limit the performance of their solutions. Public access to the training methodology for these solutions remains absent. This study implements the Type Three (T3) challenge format, which allows for training solutions on private data and guarantees reusable training methodologies. With T3, challenge organizers train a codebase provided by the participants on sequestered training data. T3 was implemented in the STOIC2021 challenge, with the goal of predicting from a computed tomography (CT) scan whether subjects had a severe COVID-19 infection, defined as intubation or death within one month. STOIC2021 consisted of a Qualification phase, where participants developed challenge solutions using 2000 publicly available CT scans, and a Final phase, where participants submitted their training methodologies with which solutions were trained on CT scans of 9724 subjects. The organizers successfully trained six of the eight Final phase submissions. The submitted codebases for training and running inference were released publicly. The winning solution obtained an area under the receiver operating characteristic curve for discerning between severe and non-severe COVID-19 of 0.815. The Final phase solutions of all finalists improved upon their Qualification phase solutions.HSUXJM-TNZF9CHSUXJM-TNZF9C
Region-guided CycleGANs for Stain Transfer in Whole Slide Images
Aug 26, 2022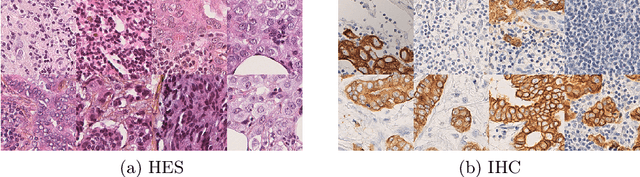
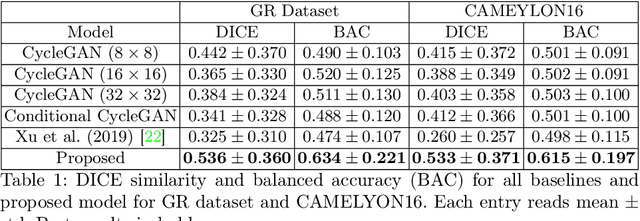
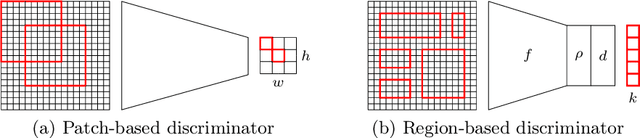
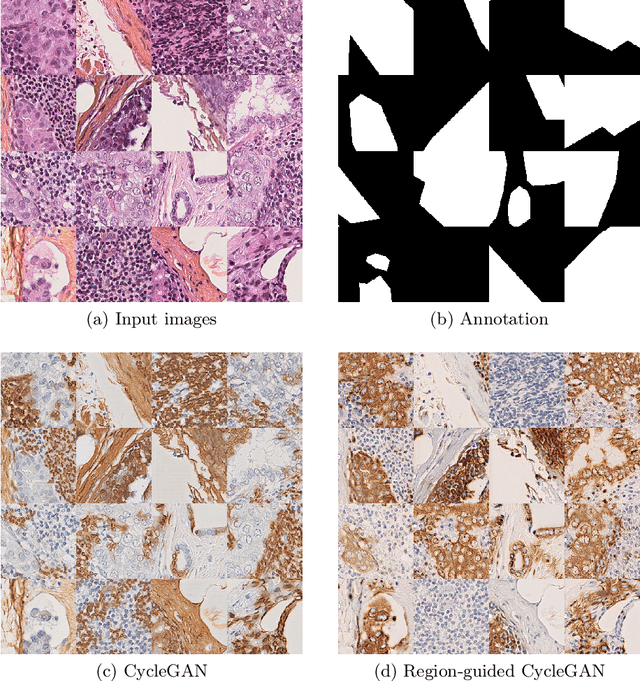
Abstract:In whole slide imaging, commonly used staining techniques based on hematoxylin and eosin (H&E) and immunohistochemistry (IHC) stains accentuate different aspects of the tissue landscape. In the case of detecting metastases, IHC provides a distinct readout that is readily interpretable by pathologists. IHC, however, is a more expensive approach and not available at all medical centers. Virtually generating IHC images from H&E using deep neural networks thus becomes an attractive alternative. Deep generative models such as CycleGANs learn a semantically-consistent mapping between two image domains, while emulating the textural properties of each domain. They are therefore a suitable choice for stain transfer applications. However, they remain fully unsupervised, and possess no mechanism for enforcing biological consistency in stain transfer. In this paper, we propose an extension to CycleGANs in the form of a region of interest discriminator. This allows the CycleGAN to learn from unpaired datasets where, in addition, there is a partial annotation of objects for which one wishes to enforce consistency. We present a use case on whole slide images, where an IHC stain provides an experimentally generated signal for metastatic cells. We demonstrate the superiority of our approach over prior art in stain transfer on histopathology tiles over two datasets. Our code and model are available at https://github.com/jcboyd/miccai2022-roigan.
MICS : Multi-steps, Inverse Consistency and Symmetric deep learning registration network
Nov 23, 2021
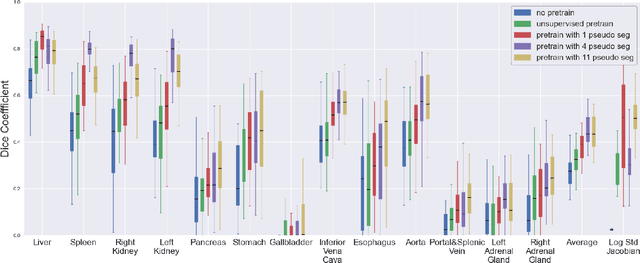
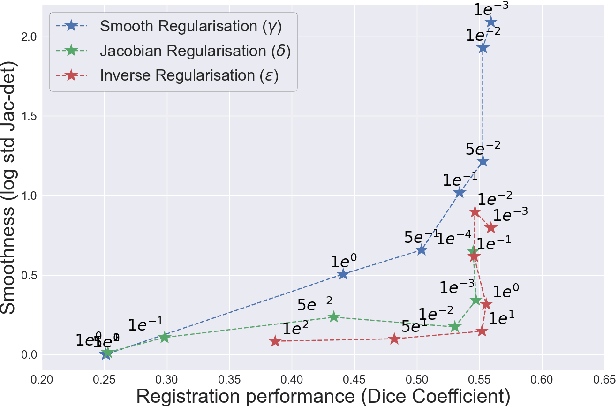

Abstract:Deformable registration consists of finding the best dense correspondence between two different images. Many algorithms have been published, but the clinical application was made difficult by the high calculation time needed to solve the optimisation problem. Deep learning overtook this limitation by taking advantage of GPU calculation and the learning process. However, many deep learning methods do not take into account desirable properties respected by classical algorithms. In this paper, we present MICS, a novel deep learning algorithm for medical imaging registration. As registration is an ill-posed problem, we focused our algorithm on the respect of different properties: inverse consistency, symmetry and orientation conservation. We also combined our algorithm with a multi-step strategy to refine and improve the deformation grid. While many approaches applied registration to brain MRI, we explored a more challenging body localisation: abdominal CT. Finally, we evaluated our method on a dataset used during the Learn2Reg challenge, allowing a fair comparison with published methods.
Self-Supervised Representation Learning using Visual Field Expansion on Digital Pathology
Sep 07, 2021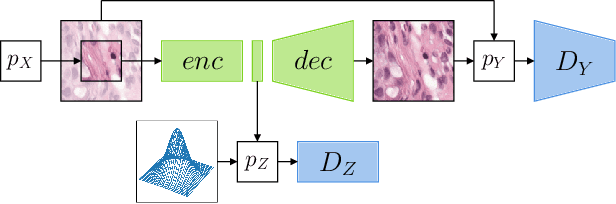
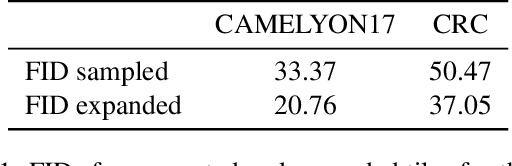
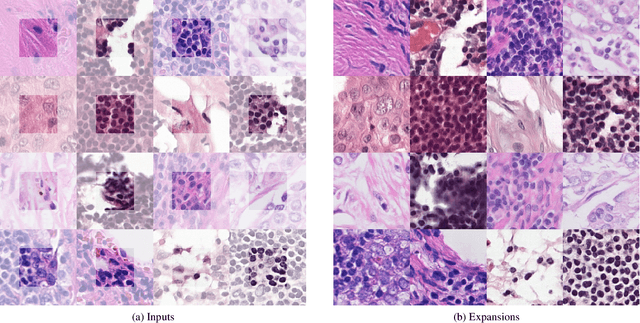
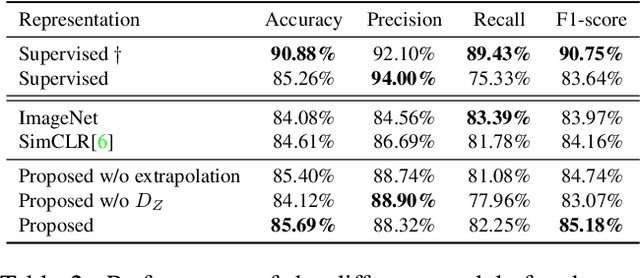
Abstract:The examination of histopathology images is considered to be the gold standard for the diagnosis and stratification of cancer patients. A key challenge in the analysis of such images is their size, which can run into the gigapixels and can require tedious screening by clinicians. With the recent advances in computational medicine, automatic tools have been proposed to assist clinicians in their everyday practice. Such tools typically process these large images by slicing them into tiles that can then be encoded and utilized for different clinical models. In this study, we propose a novel generative framework that can learn powerful representations for such tiles by learning to plausibly expand their visual field. In particular, we developed a progressively grown generative model with the objective of visual field expansion. Thus trained, our model learns to generate different tissue types with fine details, while simultaneously learning powerful representations that can be used for different clinical endpoints, all in a self-supervised way. To evaluate the performance of our model, we conducted classification experiments on CAMELYON17 and CRC benchmark datasets, comparing favorably to other self-supervised and pre-trained strategies that are commonly used in digital pathology. Our code is available at https://github.com/jcboyd/cdpath21-gan.
Deep Reinforcement Learning for L3 Slice Localization in Sarcopenia Assessment
Aug 13, 2021


Abstract:Sarcopenia is a medical condition characterized by a reduction in muscle mass and function. A quantitative diagnosis technique consists of localizing the CT slice passing through the middle of the third lumbar area (L3) and segmenting muscles at this level. In this paper, we propose a deep reinforcement learning method for accurate localization of the L3 CT slice. Our method trains a reinforcement learning agent by incentivizing it to discover the right position. Specifically, a Deep Q-Network is trained to find the best policy to follow for this problem. Visualizing the training process shows that the agent mimics the scrolling of an experienced radiologist. Extensive experiments against other state-of-the-art deep learning based methods for L3 localization prove the superiority of our technique which performs well even with a limited amount of data and annotations.
Exploring Deep Registration Latent Spaces
Jul 23, 2021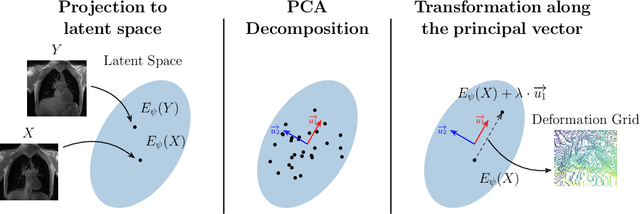
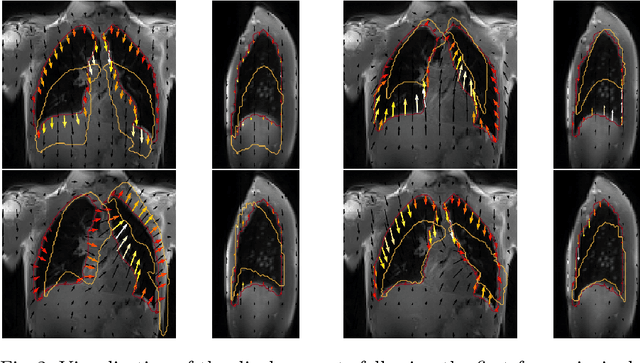
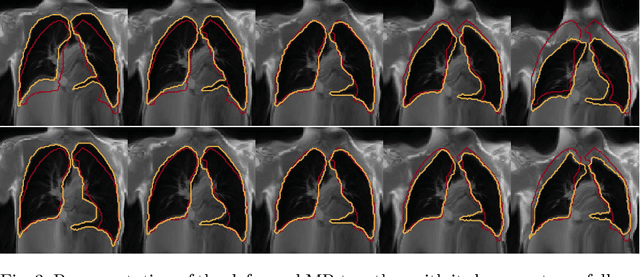
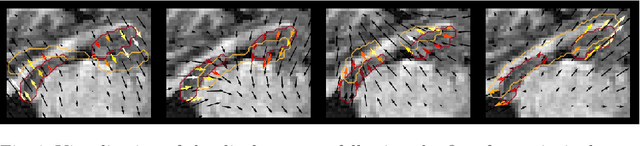
Abstract:Explainability of deep neural networks is one of the most challenging and interesting problems in the field. In this study, we investigate the topic focusing on the interpretability of deep learning-based registration methods. In particular, with the appropriate model architecture and using a simple linear projection, we decompose the encoding space, generating a new basis, and we empirically show that this basis captures various decomposed anatomically aware geometrical transformations. We perform experiments using two different datasets focusing on lungs and hippocampus MRI. We show that such an approach can decompose the highly convoluted latent spaces of registration pipelines in an orthogonal space with several interesting properties. We hope that this work could shed some light on a better understanding of deep learning-based registration methods.
Weakly supervised pan-cancer segmentation tool
May 10, 2021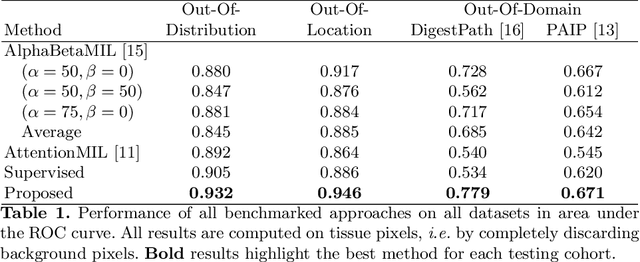
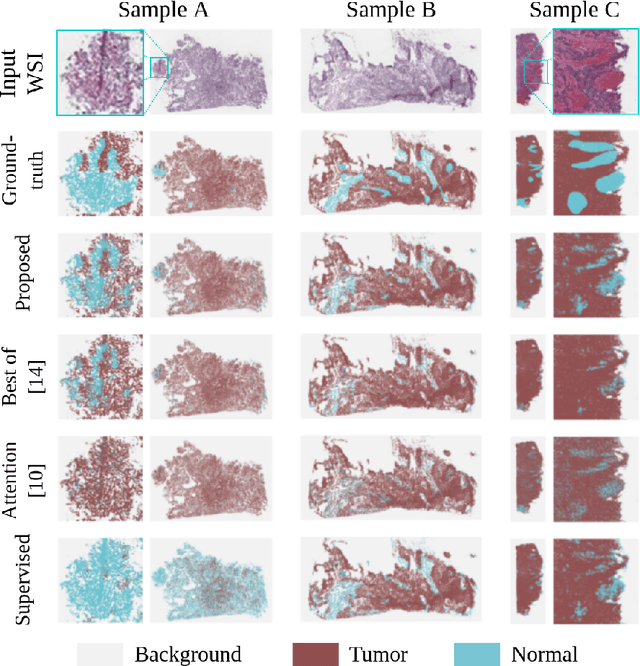
Abstract:The vast majority of semantic segmentation approaches rely on pixel-level annotations that are tedious and time consuming to obtain and suffer from significant inter and intra-expert variability. To address these issues, recent approaches have leveraged categorical annotations at the slide-level, that in general suffer from robustness and generalization. In this paper, we propose a novel weakly supervised multi-instance learning approach that deciphers quantitative slide-level annotations which are fast to obtain and regularly present in clinical routine. The extreme potentials of the proposed approach are demonstrated for tumor segmentation of solid cancer subtypes. The proposed approach achieves superior performance in out-of-distribution, out-of-location, and out-of-domain testing sets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge