Joseph Boyd
Structured State Space Models for Multiple Instance Learning in Digital Pathology
Jun 27, 2023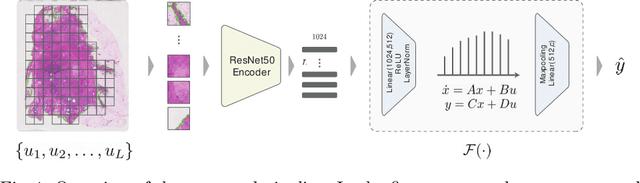
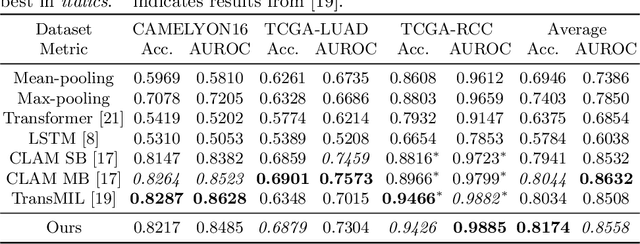

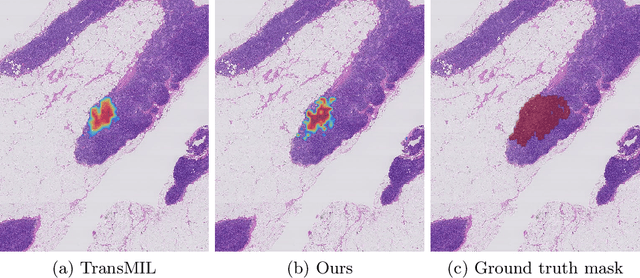
Abstract:Multiple instance learning is an ideal mode of analysis for histopathology data, where vast whole slide images are typically annotated with a single global label. In such cases, a whole slide image is modelled as a collection of tissue patches to be aggregated and classified. Common models for performing this classification include recurrent neural networks and transformers. Although powerful compression algorithms, such as deep pre-trained neural networks, are used to reduce the dimensionality of each patch, the sequences arising from whole slide images remain excessively long, routinely containing tens of thousands of patches. Structured state space models are an emerging alternative for sequence modelling, specifically designed for the efficient modelling of long sequences. These models invoke an optimal projection of an input sequence into memory units that compress the entire sequence. In this paper, we propose the use of state space models as a multiple instance learner to a variety of problems in digital pathology. Across experiments in metastasis detection, cancer subtyping, mutation classification, and multitask learning, we demonstrate the competitiveness of this new class of models with existing state of the art approaches. Our code is available at https://github.com/MICS-Lab/s4_digital_pathology.
Artifact Removal in Histopathology Images
Dec 16, 2022



Abstract:In the clinical setting of histopathology, whole-slide image (WSI) artifacts frequently arise, distorting regions of interest, and having a pernicious impact on WSI analysis. Image-to-image translation networks such as CycleGANs are in principle capable of learning an artifact removal function from unpaired data. However, we identify a surjection problem with artifact removal, and propose an weakly-supervised extension to CycleGAN to address this. We assemble a pan-cancer dataset comprising artifact and clean tiles from the TCGA database. Promising results highlight the soundness of our method.
Region-guided CycleGANs for Stain Transfer in Whole Slide Images
Aug 26, 2022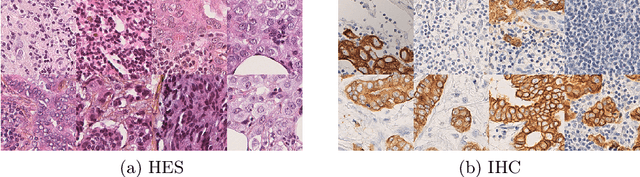
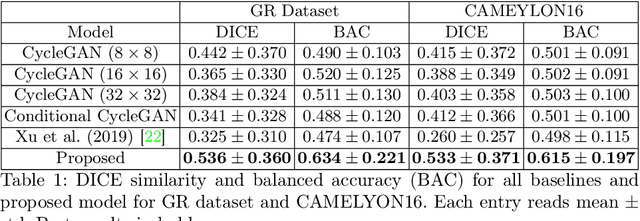
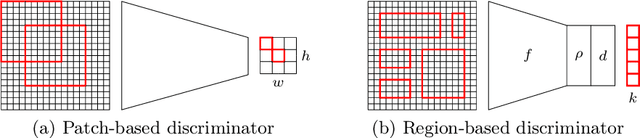
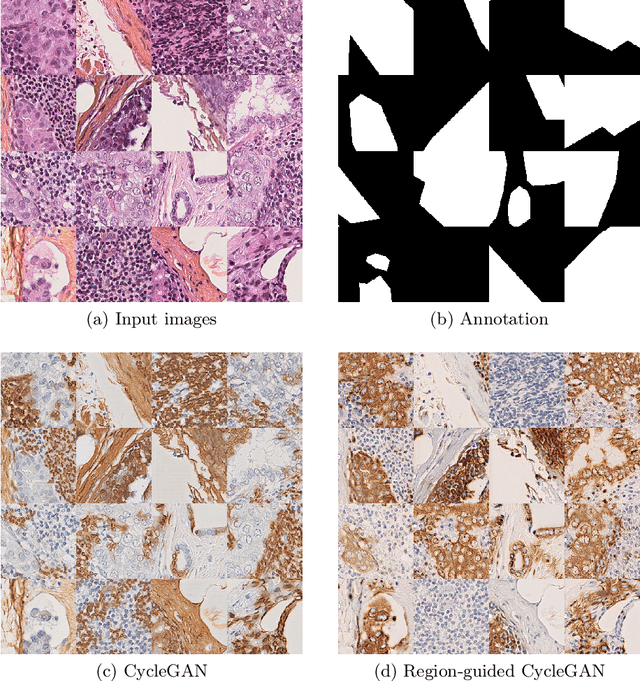
Abstract:In whole slide imaging, commonly used staining techniques based on hematoxylin and eosin (H&E) and immunohistochemistry (IHC) stains accentuate different aspects of the tissue landscape. In the case of detecting metastases, IHC provides a distinct readout that is readily interpretable by pathologists. IHC, however, is a more expensive approach and not available at all medical centers. Virtually generating IHC images from H&E using deep neural networks thus becomes an attractive alternative. Deep generative models such as CycleGANs learn a semantically-consistent mapping between two image domains, while emulating the textural properties of each domain. They are therefore a suitable choice for stain transfer applications. However, they remain fully unsupervised, and possess no mechanism for enforcing biological consistency in stain transfer. In this paper, we propose an extension to CycleGANs in the form of a region of interest discriminator. This allows the CycleGAN to learn from unpaired datasets where, in addition, there is a partial annotation of objects for which one wishes to enforce consistency. We present a use case on whole slide images, where an IHC stain provides an experimentally generated signal for metastatic cells. We demonstrate the superiority of our approach over prior art in stain transfer on histopathology tiles over two datasets. Our code and model are available at https://github.com/jcboyd/miccai2022-roigan.
Self-Supervised Representation Learning using Visual Field Expansion on Digital Pathology
Sep 07, 2021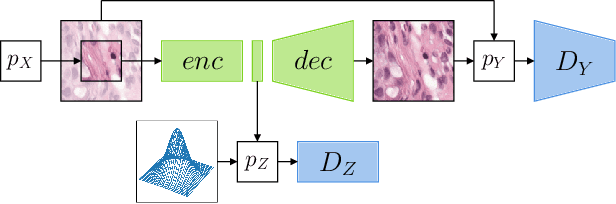
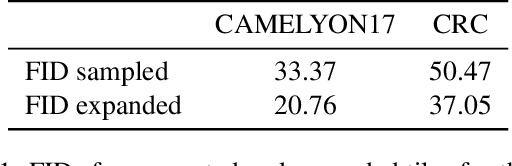
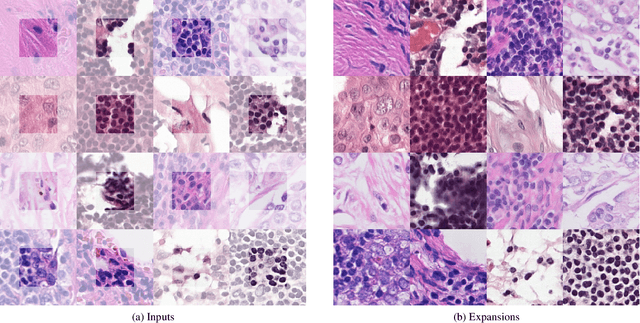
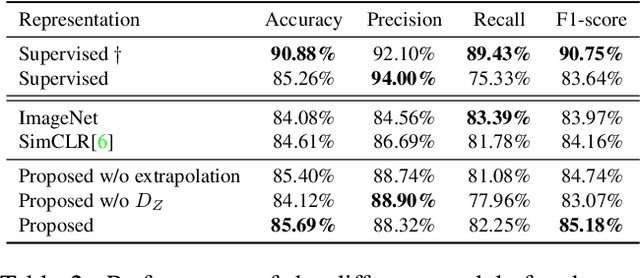
Abstract:The examination of histopathology images is considered to be the gold standard for the diagnosis and stratification of cancer patients. A key challenge in the analysis of such images is their size, which can run into the gigapixels and can require tedious screening by clinicians. With the recent advances in computational medicine, automatic tools have been proposed to assist clinicians in their everyday practice. Such tools typically process these large images by slicing them into tiles that can then be encoded and utilized for different clinical models. In this study, we propose a novel generative framework that can learn powerful representations for such tiles by learning to plausibly expand their visual field. In particular, we developed a progressively grown generative model with the objective of visual field expansion. Thus trained, our model learns to generate different tissue types with fine details, while simultaneously learning powerful representations that can be used for different clinical endpoints, all in a self-supervised way. To evaluate the performance of our model, we conducted classification experiments on CAMELYON17 and CRC benchmark datasets, comparing favorably to other self-supervised and pre-trained strategies that are commonly used in digital pathology. Our code is available at https://github.com/jcboyd/cdpath21-gan.
ASNI: Adaptive Structured Noise Injection for shallow and deep neural networks
Sep 21, 2019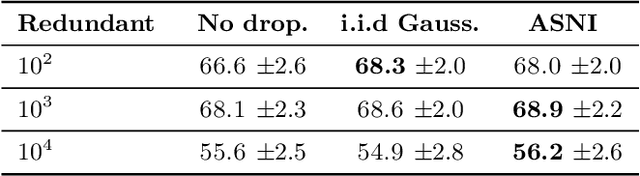
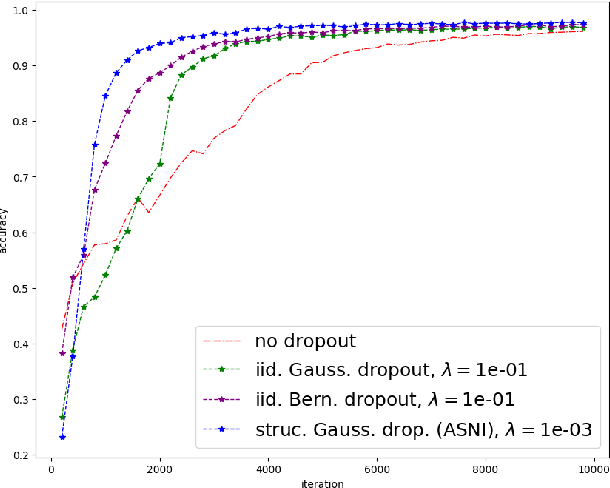
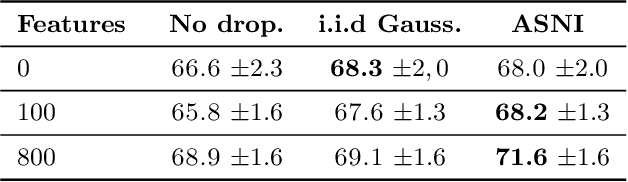
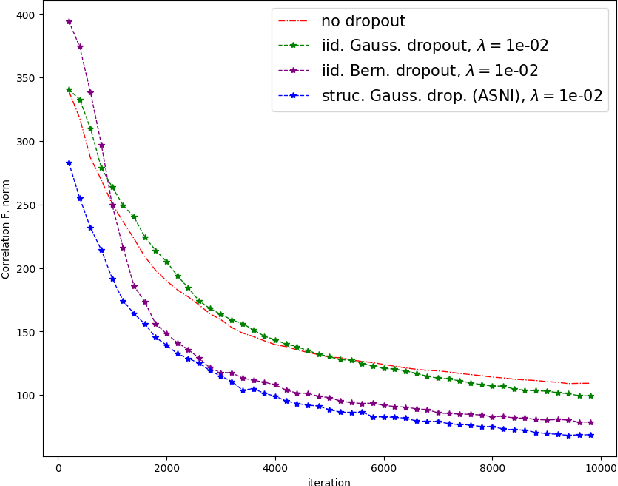
Abstract:Dropout is a regularisation technique in neural network training where unit activations are randomly set to zero with a given probability \emph{independently}. In this work, we propose a generalisation of dropout and other multiplicative noise injection schemes for shallow and deep neural networks, where the random noise applied to different units is not independent but follows a joint distribution that is either fixed or estimated during training. We provide theoretical insights on why such adaptive structured noise injection (ASNI) may be relevant, and empirically confirm that it helps boost the accuracy of simple feedforward and convolutional neural networks, disentangles the hidden layer representations, and leads to sparser representations. Our proposed method is a straightforward modification of the classical dropout and does not require additional computational overhead.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge