Marie-Pierre Revel
Advancing human-centric AI for robust X-ray analysis through holistic self-supervised learning
May 02, 2024



Abstract:AI Foundation models are gaining traction in various applications, including medical fields like radiology. However, medical foundation models are often tested on limited tasks, leaving their generalisability and biases unexplored. We present RayDINO, a large visual encoder trained by self-supervision on 873k chest X-rays. We compare RayDINO to previous state-of-the-art models across nine radiology tasks, from classification and dense segmentation to text generation, and provide an in depth analysis of population, age and sex biases of our model. Our findings suggest that self-supervision allows patient-centric AI proving useful in clinical workflows and interpreting X-rays holistically. With RayDINO and small task-specific adapters, we reach state-of-the-art results and improve generalization to unseen populations while mitigating bias, illustrating the true promise of foundation models: versatility and robustness.
Certification of Deep Learning Models for Medical Image Segmentation
Oct 05, 2023Abstract:In medical imaging, segmentation models have known a significant improvement in the past decade and are now used daily in clinical practice. However, similar to classification models, segmentation models are affected by adversarial attacks. In a safety-critical field like healthcare, certifying model predictions is of the utmost importance. Randomized smoothing has been introduced lately and provides a framework to certify models and obtain theoretical guarantees. In this paper, we present for the first time a certified segmentation baseline for medical imaging based on randomized smoothing and diffusion models. Our results show that leveraging the power of denoising diffusion probabilistic models helps us overcome the limits of randomized smoothing. We conduct extensive experiments on five public datasets of chest X-rays, skin lesions, and colonoscopies, and empirically show that we are able to maintain high certified Dice scores even for highly perturbed images. Our work represents the first attempt to certify medical image segmentation models, and we aspire for it to set a foundation for future benchmarks in this crucial and largely uncharted area.
Towards Better Certified Segmentation via Diffusion Models
Jun 16, 2023



Abstract:The robustness of image segmentation has been an important research topic in the past few years as segmentation models have reached production-level accuracy. However, like classification models, segmentation models can be vulnerable to adversarial perturbations, which hinders their use in critical-decision systems like healthcare or autonomous driving. Recently, randomized smoothing has been proposed to certify segmentation predictions by adding Gaussian noise to the input to obtain theoretical guarantees. However, this method exhibits a trade-off between the amount of added noise and the level of certification achieved. In this paper, we address the problem of certifying segmentation prediction using a combination of randomized smoothing and diffusion models. Our experiments show that combining randomized smoothing and diffusion models significantly improves certified robustness, with results indicating a mean improvement of 21 points in accuracy compared to previous state-of-the-art methods on Pascal-Context and Cityscapes public datasets. Our method is independent of the selected segmentation model and does not need any additional specialized training procedure.
Deep Reinforcement Learning for L3 Slice Localization in Sarcopenia Assessment
Aug 13, 2021


Abstract:Sarcopenia is a medical condition characterized by a reduction in muscle mass and function. A quantitative diagnosis technique consists of localizing the CT slice passing through the middle of the third lumbar area (L3) and segmenting muscles at this level. In this paper, we propose a deep reinforcement learning method for accurate localization of the L3 CT slice. Our method trains a reinforcement learning agent by incentivizing it to discover the right position. Specifically, a Deep Q-Network is trained to find the best policy to follow for this problem. Visualizing the training process shows that the agent mimics the scrolling of an experienced radiologist. Extensive experiments against other state-of-the-art deep learning based methods for L3 localization prove the superiority of our technique which performs well even with a limited amount of data and annotations.
Exploring Deep Registration Latent Spaces
Jul 23, 2021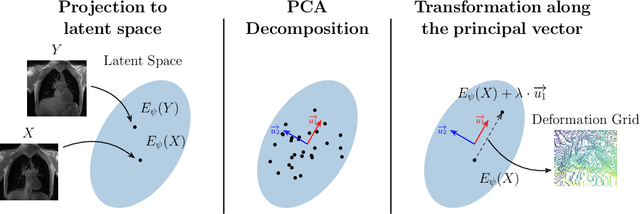
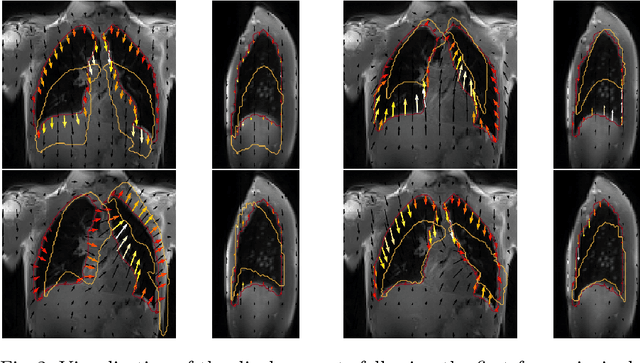
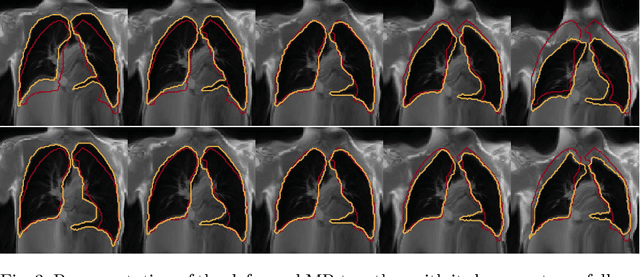
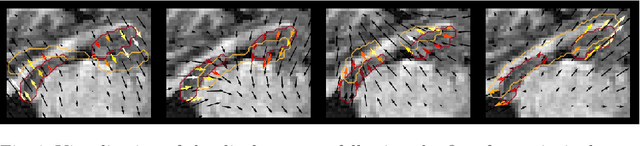
Abstract:Explainability of deep neural networks is one of the most challenging and interesting problems in the field. In this study, we investigate the topic focusing on the interpretability of deep learning-based registration methods. In particular, with the appropriate model architecture and using a simple linear projection, we decompose the encoding space, generating a new basis, and we empirically show that this basis captures various decomposed anatomically aware geometrical transformations. We perform experiments using two different datasets focusing on lungs and hippocampus MRI. We show that such an approach can decompose the highly convoluted latent spaces of registration pipelines in an orthogonal space with several interesting properties. We hope that this work could shed some light on a better understanding of deep learning-based registration methods.
AI-Driven CT-based quantification, staging and short-term outcome prediction of COVID-19 pneumonia
Apr 20, 2020Abstract:Chest computed tomography (CT) is widely used for the management of Coronavirus disease 2019 (COVID-19) pneumonia because of its availability and rapidity. The standard of reference for confirming COVID-19 relies on microbiological tests but these tests might not be available in an emergency setting and their results are not immediately available, contrary to CT. In addition to its role for early diagnosis, CT has a prognostic role by allowing visually evaluating the extent of COVID-19 lung abnormalities. The objective of this study is to address prediction of short-term outcomes, especially need for mechanical ventilation. In this multi-centric study, we propose an end-to-end artificial intelligence solution for automatic quantification and prognosis assessment by combining automatic CT delineation of lung disease meeting performance of experts and data-driven identification of biomarkers for its prognosis. AI-driven combination of variables with CT-based biomarkers offers perspectives for optimal patient management given the shortage of intensive care beds and ventilators.
Linear and Deformable Image Registration with 3D Convolutional Neural Networks
Sep 13, 2018



Abstract:Image registration and in particular deformable registration methods are pillars of medical imaging. Inspired by the recent advances in deep learning, we propose in this paper, a novel convolutional neural network architecture that couples linear and deformable registration within a unified architecture endowed with near real-time performance. Our framework is modular with respect to the global transformation component, as well as with respect to the similarity function while it guarantees smooth displacement fields. We evaluate the performance of our network on the challenging problem of MRI lung registration, and demonstrate superior performance with respect to state of the art elastic registration methods. The proposed deformation (between inspiration & expiration) was considered within a clinically relevant task of interstitial lung disease (ILD) classification and showed promising results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge