Naruki Yoshikawa
RoboCulture: A Robotics Platform for Automated Biological Experimentation
May 20, 2025Abstract:Automating biological experimentation remains challenging due to the need for millimeter-scale precision, long and multi-step experiments, and the dynamic nature of living systems. Current liquid handlers only partially automate workflows, requiring human intervention for plate loading, tip replacement, and calibration. Industrial solutions offer more automation but are costly and lack the flexibility needed in research settings. Meanwhile, research in autonomous robotics has yet to bridge the gap for long-duration, failure-sensitive biological experiments. We introduce RoboCulture, a cost-effective and flexible platform that uses a general-purpose robotic manipulator to automate key biological tasks. RoboCulture performs liquid handling, interacts with lab equipment, and leverages computer vision for real-time decisions using optical density-based growth monitoring. We demonstrate a fully autonomous 15-hour yeast culture experiment where RoboCulture uses vision and force feedback and a modular behavior tree framework to robustly execute, monitor, and manage experiments.
Accelerating Discovery in Natural Science Laboratories with AI and Robotics: Perspectives and Challenges from the 2024 IEEE ICRA Workshop, Yokohama, Japan
Jan 12, 2025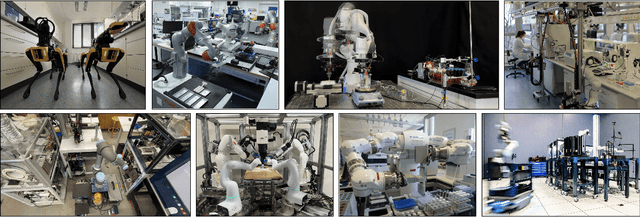
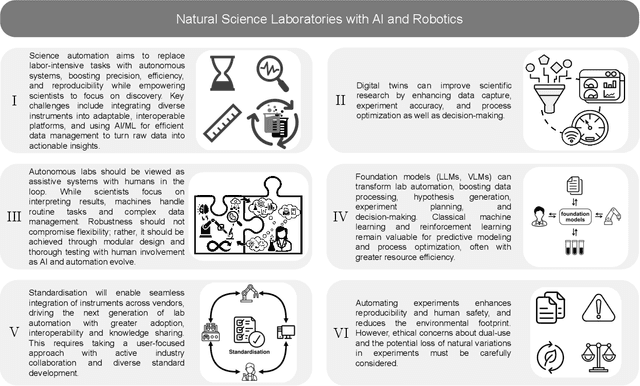
Abstract:Science laboratory automation enables accelerated discovery in life sciences and materials. However, it requires interdisciplinary collaboration to address challenges such as robust and flexible autonomy, reproducibility, throughput, standardization, the role of human scientists, and ethics. This article highlights these issues, reflecting perspectives from leading experts in laboratory automation across different disciplines of the natural sciences.
ORGANA: A Robotic Assistant for Automated Chemistry Experimentation and Characterization
Jan 13, 2024



Abstract:Chemistry experimentation is often resource- and labor-intensive. Despite the many benefits incurred by the integration of advanced and special-purpose lab equipment, many aspects of experimentation are still manually conducted by chemists, for example, polishing an electrode in electrochemistry experiments. Traditional lab automation infrastructure faces challenges when it comes to flexibly adapting to new chemistry experiments. To address this issue, we propose a human-friendly and flexible robotic system, ORGANA, that automates a diverse set of chemistry experiments. It is capable of interacting with chemists in the lab through natural language, using Large Language Models (LLMs). ORGANA keeps scientists informed by providing timely reports that incorporate statistical analyses. Additionally, it actively engages with users when necessary for disambiguation or troubleshooting. ORGANA can reason over user input to derive experiment goals, and plan long sequences of both high-level tasks and low-level robot actions while using feedback from the visual perception of the environment. It also supports scheduling and parallel execution for experiments that require resource allocation and coordination between multiple robots and experiment stations. We show that ORGANA successfully conducts a diverse set of chemistry experiments, including solubility assessment, pH measurement, recrystallization, and electrochemistry experiments. For the latter, we show that ORGANA robustly executes a long-horizon plan, comprising 19 steps executed in parallel, to characterize the electrochemical properties of quinone derivatives, a class of molecules used in rechargeable flow batteries. Our user study indicates that ORGANA significantly improves many aspects of user experience while reducing their physical workload. More details about ORGANA can be found at https://ac-rad.github.io/organa/.
Errors are Useful Prompts: Instruction Guided Task Programming with Verifier-Assisted Iterative Prompting
Mar 24, 2023Abstract:Generating low-level robot task plans from high-level natural language instructions remains a challenging problem. Although large language models have shown promising results in generating plans, the accuracy of the output remains unverified. Furthermore, the lack of domain-specific language data poses a limitation on the applicability of these models. In this paper, we propose CLAIRIFY, a novel approach that combines automatic iterative prompting with program verification to ensure programs written in data-scarce domain-specific language are syntactically valid and incorporate environment constraints. Our approach provides effective guidance to the language model on generating structured-like task plans by incorporating any errors as feedback, while the verifier ensures the syntactic accuracy of the generated plans. We demonstrate the effectiveness of CLAIRIFY in planning chemistry experiments by achieving state-of-the-art results. We also show that the generated plans can be executed on a real robot by integrating them with a task and motion planner.
An Adaptive Robotics Framework for Chemistry Lab Automation
Dec 19, 2022Abstract:In the process of materials discovery, chemists currently need to perform many laborious, time-consuming, and often dangerous lab experiments. To accelerate this process, we propose a framework for robots to assist chemists by performing lab experiments autonomously. The solution allows a general-purpose robot to perform diverse chemistry experiments and efficiently make use of available lab tools. Our system can load high-level descriptions of chemistry experiments, perceive a dynamic workspace, and autonomously plan the required actions and motions to perform the given chemistry experiments with common tools found in the existing lab environment. Our architecture uses a modified PDDLStream solver for integrated task and constrained motion planning, which generates plans and motions that are guaranteed to be safe by preventing collisions and spillage. We present a modular framework that can scale to many different experiments, actions, and lab tools. In this work, we demonstrate the utility of our framework on three pouring skills and two foundational chemical experiments for materials synthesis: solubility and recrystallization. More experiments and updated evaluations can be found at https://ac-rad.github.io/arc-icra2023.
Assigning Confidence to Molecular Property Prediction
Feb 23, 2021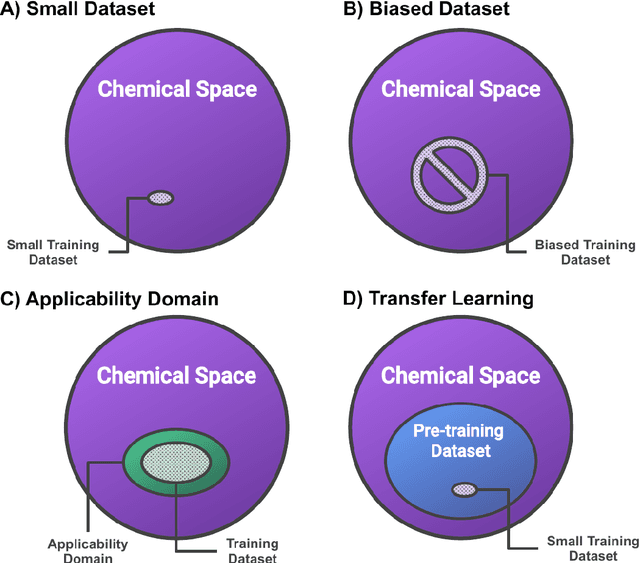
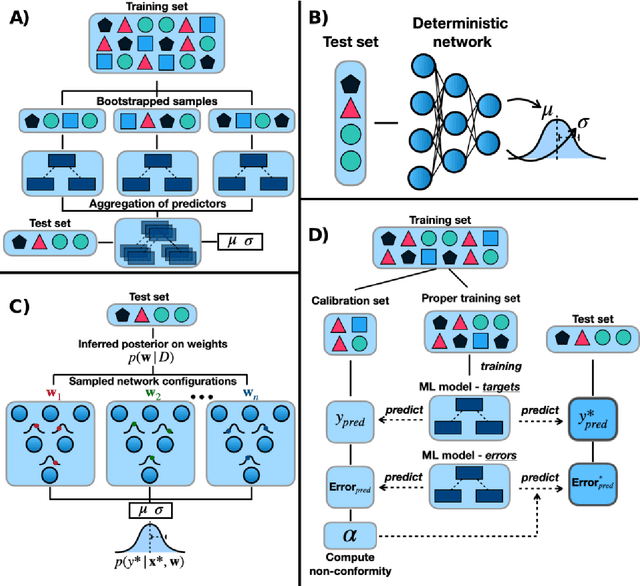
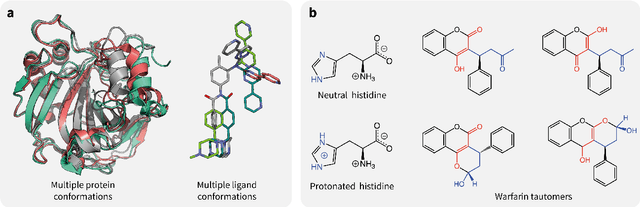
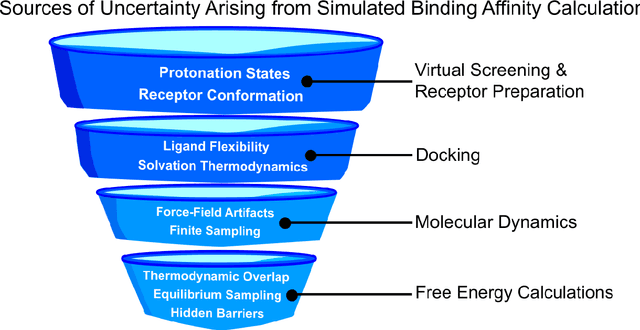
Abstract:Introduction: Computational modeling has rapidly advanced over the last decades, especially to predict molecular properties for chemistry, material science and drug design. Recently, machine learning techniques have emerged as a powerful and cost-effective strategy to learn from existing datasets and perform predictions on unseen molecules. Accordingly, the explosive rise of data-driven techniques raises an important question: What confidence can be assigned to molecular property predictions and what techniques can be used for that purpose? Areas covered: In this work, we discuss popular strategies for predicting molecular properties relevant to drug design, their corresponding uncertainty sources and methods to quantify uncertainty and confidence. First, our considerations for assessing confidence begin with dataset bias and size, data-driven property prediction and feature design. Next, we discuss property simulation via molecular docking, and free-energy simulations of binding affinity in detail. Lastly, we investigate how these uncertainties propagate to generative models, as they are usually coupled with property predictors. Expert opinion: Computational techniques are paramount to reduce the prohibitive cost and timing of brute-force experimentation when exploring the enormous chemical space. We believe that assessing uncertainty in property prediction models is essential whenever closed-loop drug design campaigns relying on high-throughput virtual screening are deployed. Accordingly, considering sources of uncertainty leads to better-informed experimental validations, more reliable predictions and to more realistic expectations of the entire workflow. Overall, this increases confidence in the predictions and designs and, ultimately, accelerates drug design.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge