Hatem Fakhruldeen
MATTERIX: toward a digital twin for robotics-assisted chemistry laboratory automation
Jan 19, 2026Abstract:Accelerated materials discovery is critical for addressing global challenges. However, developing new laboratory workflows relies heavily on real-world experimental trials, and this can hinder scalability because of the need for numerous physical make-and-test iterations. Here we present MATTERIX, a multiscale, graphics processing unit-accelerated robotic simulation framework designed to create high-fidelity digital twins of chemistry laboratories, thus accelerating workflow development. This multiscale digital twin simulates robotic physical manipulation, powder and liquid dynamics, device functionalities, heat transfer and basic chemical reaction kinetics. This is enabled by integrating realistic physics simulation and photorealistic rendering with a modular graphics processing unit-accelerated semantics engine, which models logical states and continuous behaviors to simulate chemistry workflows across different levels of abstraction. MATTERIX streamlines the creation of digital twin environments through open-source asset libraries and interfaces, while enabling flexible workflow design via hierarchical plan definition and a modular skill library that incorporates learning-based methods. Our approach demonstrates sim-to-real transfer in robotic chemistry setups, reducing reliance on costly real-world experiments and enabling the testing of hypothetical automated workflows in silico. The project website is available at https://accelerationconsortium.github.io/Matterix/ .
PREVENT: Proactive Risk Evaluation and Vigilant Execution of Tasks for Mobile Robotic Chemists using Multi-Modal Behavior Trees
Oct 24, 2025Abstract:Mobile robotic chemists are a fast growing trend in the field of chemistry and materials research. However, so far these mobile robots lack workflow awareness skills. This poses the risk that even a small anomaly, such as an improperly capped sample vial could disrupt the entire workflow. This wastes time, and resources, and could pose risks to human researchers, such as exposure to toxic materials. Existing perception mechanisms can be used to predict anomalies but they often generate excessive false positives. This may halt workflow execution unnecessarily, requiring researchers to intervene and to resume the workflow when no problem actually exists, negating the benefits of autonomous operation. To address this problem, we propose PREVENT a system comprising navigation and manipulation skills based on a multimodal Behavior Tree (BT) approach that can be integrated into existing software architectures with minimal modifications. Our approach involves a hierarchical perception mechanism that exploits AI techniques and sensory feedback through Dexterous Vision and Navigational Vision cameras and an IoT gas sensor module for execution-related decision-making. Experimental evaluations show that the proposed approach is comparatively efficient and completely avoids both false negatives and false positives when tested in simulated risk scenarios within our robotic chemistry workflow. The results also show that the proposed multi-modal perception skills achieved deployment accuracies that were higher than the average of the corresponding uni-modal skills, both for navigation and for manipulation.
Chemist Eye: A Visual Language Model-Powered System for Safety Monitoring and Robot Decision-Making in Self-Driving Laboratories
Aug 07, 2025Abstract:The integration of robotics and automation into self-driving laboratories (SDLs) can introduce additional safety complexities, in addition to those that already apply to conventional research laboratories. Personal protective equipment (PPE) is an essential requirement for ensuring the safety and well-being of workers in laboratories, self-driving or otherwise. Fires are another important risk factor in chemical laboratories. In SDLs, fires that occur close to mobile robots, which use flammable lithium batteries, could have increased severity. Here, we present Chemist Eye, a distributed safety monitoring system designed to enhance situational awareness in SDLs. The system integrates multiple stations equipped with RGB, depth, and infrared cameras, designed to monitor incidents in SDLs. Chemist Eye is also designed to spot workers who have suffered a potential accident or medical emergency, PPE compliance and fire hazards. To do this, Chemist Eye uses decision-making driven by a vision-language model (VLM). Chemist Eye is designed for seamless integration, enabling real-time communication with robots. Based on the VLM recommendations, the system attempts to drive mobile robots away from potential fire locations, exits, or individuals not wearing PPE, and issues audible warnings where necessary. It also integrates with third-party messaging platforms to provide instant notifications to lab personnel. We tested Chemist Eye with real-world data from an SDL equipped with three mobile robots and found that the spotting of possible safety hazards and decision-making performances reached 97 % and 95 %, respectively.
An Open-source Capping Machine Suitable for Confined Spaces
Jun 04, 2025Abstract:In the context of self-driving laboratories (SDLs), ensuring automated and error-free capping is crucial, as it is a ubiquitous step in sample preparation. Automated capping in SDLs can occur in both large and small workspaces (e.g., inside a fume hood). However, most commercial capping machines are designed primarily for large spaces and are often too bulky for confined environments. Moreover, many commercial products are closed-source, which can make their integration into fully autonomous workflows difficult. This paper introduces an open-source capping machine suitable for compact spaces, which also integrates a vision system that recognises capping failure. The capping and uncapping processes are repeated 100 times each to validate the machine's design and performance. As a result, the capping machine reached a 100 % success rate for capping and uncapping. Furthermore, the machine sealing capacities are evaluated by capping 12 vials filled with solvents of different vapour pressures: water, ethanol and acetone. The vials are then weighed every 3 hours for three days. The machine's performance is benchmarked against an industrial capping machine (a Chemspeed station) and manual capping. The vials capped with the prototype lost 0.54 % of their content weight on average per day, while the ones capped with the Chemspeed and manually lost 0.0078 % and 0.013 %, respectively. The results show that the capping machine is a reasonable alternative to industrial and manual capping, especially when space and budget are limitations in SDLs.
Accelerating Discovery in Natural Science Laboratories with AI and Robotics: Perspectives and Challenges from the 2024 IEEE ICRA Workshop, Yokohama, Japan
Jan 12, 2025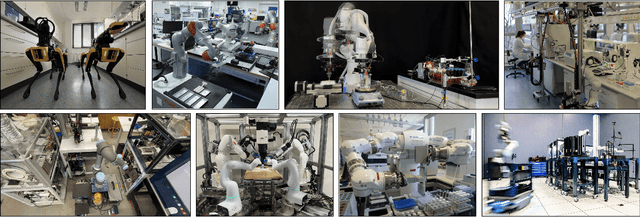
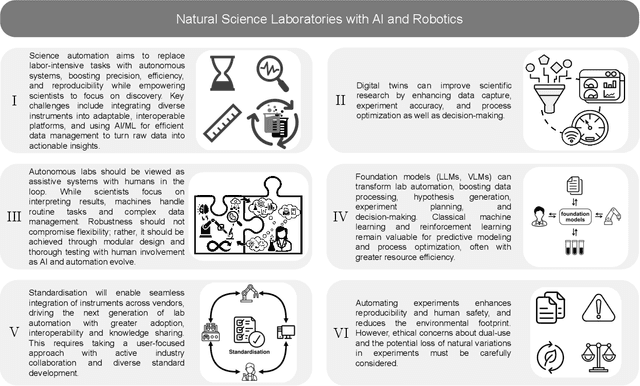
Abstract:Science laboratory automation enables accelerated discovery in life sciences and materials. However, it requires interdisciplinary collaboration to address challenges such as robust and flexible autonomy, reproducibility, throughput, standardization, the role of human scientists, and ethics. This article highlights these issues, reflecting perspectives from leading experts in laboratory automation across different disciplines of the natural sciences.
Powder-Bot: A Modular Autonomous Multi-Robot Workflow for Powder X-Ray Diffraction
Sep 01, 2023Abstract:Powder X-ray diffraction (PXRD) is a key technique for the structural characterisation of solid-state materials, but compared with tasks such as liquid handling, its end-to-end automation is highly challenging. This is because coupling PXRD experiments with crystallisation comprises multiple solid handling steps that include sample recovery, sample preparation by grinding, sample mounting and, finally, collection of X-ray diffraction data. Each of these steps has individual technical challenges from an automation perspective, and hence no commercial instrument exists that can grow crystals, process them into a powder, mount them in a diffractometer, and collect PXRD data in an autonomous, closed-loop way. Here we present an automated robotic workflow to carry out autonomous PXRD experiments. The PXRD data collected for polymorphs of small organic compounds is comparable to that collected under the same conditions manually. Beyond accelerating PXRD experiments, this workflow involves 13 component steps and integrates three different types of robots, each from a separate supplier, illustrating the power of flexible, modular automation in complex, multitask laboratories.
Accelerating Laboratory Automation Through Robot Skill Learning For Sample Scraping
Sep 29, 2022
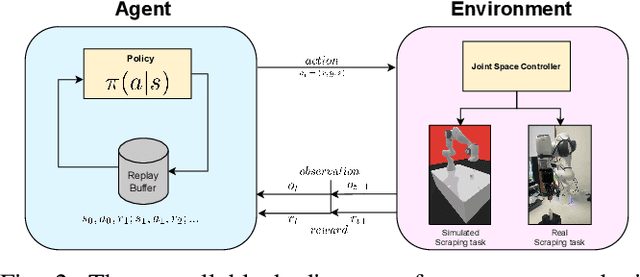


Abstract:The potential use of robotics for laboratory experiments offers an attractive route to alleviate scientists from tedious tasks while accelerating the process of obtaining new materials, where topical issues such as climate change and disease risks worldwide would greatly benefit. While some experimental workflows can already benefit from automation, it is common that sample preparation is still carried out manually due to the high level of motor function required when dealing with heterogeneous systems, e.g., different tools, chemicals, and glassware. A fundamental workflow in chemical fields is crystallisation, where one application is polymorph screening, i.e., obtaining a three dimensional molecular structure from a crystal. For this process, it is of utmost importance to recover as much of the sample as possible since synthesising molecules is both costly in time and money. To this aim, chemists have to scrape vials to retrieve sample contents prior to imaging plate transfer. Automating this process is challenging as it goes beyond robotic insertion tasks due to a fundamental requirement of having to execute fine-granular movements within a constrained environment that is the sample vial. Motivated by how human chemists carry out this process of scraping powder from vials, our work proposes a model-free reinforcement learning method for learning a scraping policy, leading to a fully autonomous sample scraping procedure. To realise that, we first create a simulation environment with a Panda Franka Emika robot using a laboratory scraper which is inserted into a simulated vial, to demonstrate how a scraping policy can be learned successfully. We then evaluate our method on a real robotic manipulator in laboratory settings, and show that our method can autonomously scrape powder across various setups.
ARChemist: Autonomous Robotic Chemistry System Architecture
Apr 28, 2022



Abstract:Automated laboratory experiments have the potential to propel new discoveries, while increasing reproducibility and improving scientists' safety when handling dangerous materials. However, many automated laboratory workflows have not fully leveraged the remarkable advancements in robotics and digital lab equipment. As a result, most robotic systems used in the labs are programmed specifically for a single experiment, often relying on proprietary architectures or using unconventional hardware. In this work, we tackle this problem by proposing a novel robotic system architecture specifically designed with and for chemists, which allows the scientist to easily reconfigure their setup for new experiments. Specifically, the system's strength is its ability to combine together heterogeneous robotic platforms with standard laboratory equipment to create different experimental setups. Finally, we show how the architecture can be used for specific laboratory experiments through case studies such as solubility screening and crystallisation.
SOLIS: Autonomous Solubility Screening using Deep Neural Networks
Mar 18, 2022



Abstract:Accelerating material discovery has tremendous societal and industrial impact, particularly for pharmaceuticals and clean energy production. Many experimental instruments have some degree of automation, facilitating continuous running and higher throughput. However, it is common that sample preparation is still carried out manually. This can result in researchers spending a significant amount of their time on repetitive tasks, which introduces errors and can prohibit production of statistically relevant data. Crystallisation experiments are common in many chemical fields, both for purification and in polymorph screening experiments. The initial step often involves a solubility screen of the molecule; that is, understanding whether molecular compounds have dissolved in a particular solvent. This usually can be time consuming and work intensive. Moreover, accurate knowledge of the precise solubility limit of the molecule is often not required, and simply measuring a threshold of solubility in each solvent would be sufficient. To address this, we propose a novel cascaded deep model that is inspired by how a human chemist would visually assess a sample to determine whether the solid has completely dissolved in the solution. In this paper, we design, develop, and evaluate the first fully autonomous solubility screening framework, which leverages state-of-the-art methods for image segmentation and convolutional neural networks for image classification. To realise that, we first create a dataset comprising different molecules and solvents, which is collected in a real-world chemistry laboratory. We then evaluated our method on the data recorded through an eye-in-hand camera mounted on a seven degree-of-freedom robotic manipulator, and show that our model can achieve 99.13% test accuracy across various setups.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge