Kris Thielemans
Accelerated Convergent Motion Compensated Image Reconstruction
Oct 14, 2024


Abstract:Motion correction aims to prevent motion artefacts which may be caused by respiration, heartbeat, or head movements for example. In a preliminary step, the measured data is divided in gates corresponding to motion states, and displacement maps from a reference state to each motion state are estimated. One common technique to perform motion correction is the motion compensated image reconstruction framework, where the displacement maps are integrated into the forward model corresponding to gated data. For standard algorithms, the computational cost per iteration increases linearly with the number of gates. In order to accelerate the reconstruction, we propose the use of a randomized and convergent algorithm whose per iteration computational cost scales constantly with the number of gates. We show improvement on theoretical rates of convergence and observe the predicted speed-up on two synthetic datasets corresponding to rigid and non-rigid motion.
Resolving Variable Respiratory Motion From Unsorted 4D Computed Tomography
Jun 30, 2024
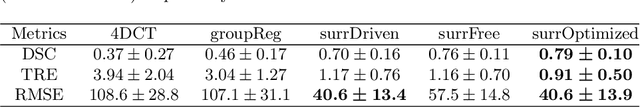


Abstract:4D Computed Tomography (4DCT) is widely used for many clinical applications such as radiotherapy treatment planning, PET and ventilation imaging. However, common 4DCT methods reconstruct multiple breath cycles into a single, arbitrary breath cycle which can lead to various artefacts, impacting the downstream clinical applications. Surrogate driven motion models can estimate continuous variable motion across multiple cycles based on CT segments `unsorted' from 4DCT, but it requires respiration surrogate signals with strong correlation to the internal motion, which are not always available. The method proposed in this study eliminates such dependency by adapting the hyper-gradient method to the optimization of surrogate signals as hyper-parameters, while achieving better or comparable performance, as demonstrated on digital phantom simulations and real patient data. Our method produces a high-quality motion-compensated image together with estimates of the motion, including breath-to-breath variability, throughout the image acquisition. Our method has the potential to improve downstream clinical applications, and also enables retrospective analysis of open access 4DCT dataset where no respiration signals are stored. Code is avaibale at https://github.com/Yuliang-Huang/4DCT-irregular-motion.
Stochastic Optimisation Framework using the Core Imaging Library and Synergistic Image Reconstruction Framework for PET Reconstruction
Jun 21, 2024Abstract:We introduce a stochastic framework into the open--source Core Imaging Library (CIL) which enables easy development of stochastic algorithms. Five such algorithms from the literature are developed, Stochastic Gradient Descent, Stochastic Average Gradient (-Am\'elior\'e), (Loopless) Stochastic Variance Reduced Gradient. We showcase the functionality of the framework with a comparative study against a deterministic algorithm on a simulated 2D PET dataset, with the use of the open-source Synergistic Image Reconstruction Framework. We observe that stochastic optimisation methods can converge in fewer passes of the data than a standard deterministic algorithm.
Score-Based Generative Models for PET Image Reconstruction
Aug 27, 2023



Abstract:Score-based generative models have demonstrated highly promising results for medical image reconstruction tasks in magnetic resonance imaging or computed tomography. However, their application to Positron Emission Tomography (PET) is still largely unexplored. PET image reconstruction involves a variety of challenges, including Poisson noise with high variance and a wide dynamic range. To address these challenges, we propose several PET-specific adaptations of score-based generative models. The proposed framework is developed for both 2D and 3D PET. In addition, we provide an extension to guided reconstruction using magnetic resonance images. We validate the approach through extensive 2D and 3D $\textit{in-silico}$ experiments with a model trained on patient-realistic data without lesions, and evaluate on data without lesions as well as out-of-distribution data with lesions. This demonstrates the proposed method's robustness and significant potential for improved PET reconstruction.
Artificial Intelligence in PET: an Industry Perspective
Jul 14, 2021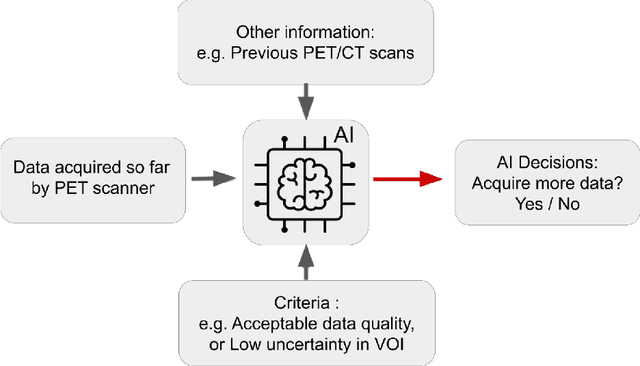
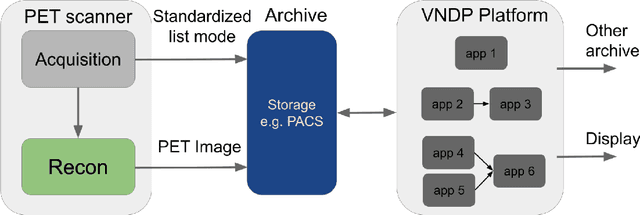
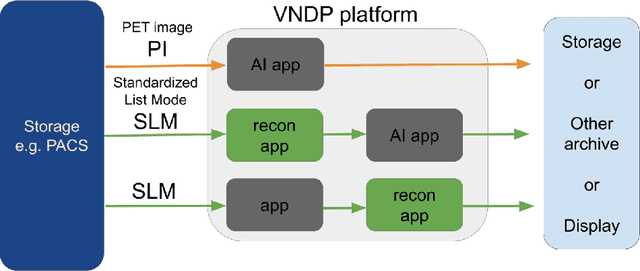
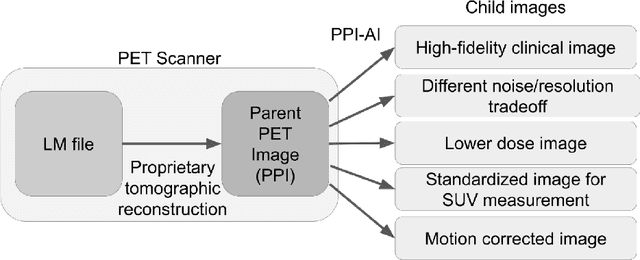
Abstract:Artificial intelligence (AI) has significant potential to positively impact and advance medical imaging, including positron emission tomography (PET) imaging applications. AI has the ability to enhance and optimize all aspects of the PET imaging chain from patient scheduling, patient setup, protocoling, data acquisition, detector signal processing, reconstruction, image processing and interpretation. AI poses industry-specific challenges which will need to be addressed and overcome to maximize the future potentials of AI in PET. This paper provides an overview of these industry-specific challenges for the development, standardization, commercialization, and clinical adoption of AI, and explores the potential enhancements to PET imaging brought on by AI in the near future. In particular, the combination of on-demand image reconstruction, AI, and custom designed data processing workflows may open new possibilities for innovation which would positively impact the industry and ultimately patients.
Improved MR to CT synthesis for PET/MR attenuation correction using Imitation Learning
Aug 27, 2019Abstract:The ability to synthesise Computed Tomography images - commonly known as pseudo CT, or pCT - from MRI input data is commonly assessed using an intensity-wise similarity, such as an L2-norm between the ground truth CT and the pCT. However, given that the ultimate purpose is often to use the pCT as an attenuation map ($\mu$-map) in Positron Emission Tomography Magnetic Resonance Imaging (PET/MRI), minimising the error between pCT and CT is not necessarily optimal. The main objective should be to predict a pCT that, when used as $\mu$-map, reconstructs a pseudo PET (pPET) which is as close as possible to the gold standard PET. To this end, we propose a novel multi-hypothesis deep learning framework that generates pCTs by minimising a combination of the pixel-wise error between pCT and CT and a proposed metric-loss that itself is represented by a convolutional neural network (CNN) and aims to minimise subsequent PET residuals. The model is trained on a database of 400 paired MR/CT/PET image slices. Quantitative results show that the network generates pCTs that seem less accurate when evaluating the Mean Absolute Error on the pCT (69.68HU) compared to a baseline CNN (66.25HU), but lead to significant improvement in the PET reconstruction - 115a.u. compared to baseline 140a.u.
Deep Boosted Regression for MR to CT Synthesis
Aug 22, 2018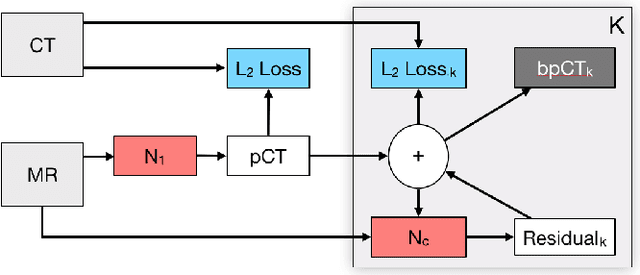
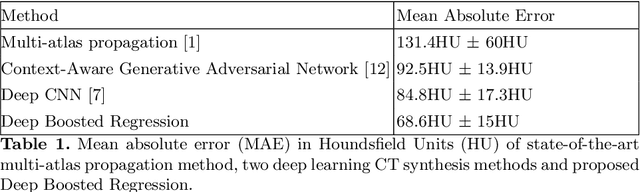
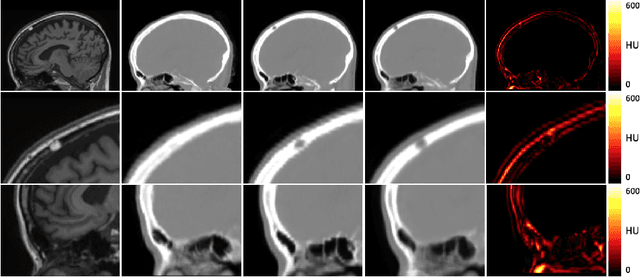
Abstract:Attenuation correction is an essential requirement of positron emission tomography (PET) image reconstruction to allow for accurate quantification. However, attenuation correction is particularly challenging for PET-MRI as neither PET nor magnetic resonance imaging (MRI) can directly image tissue attenuation properties. MRI-based computed tomography (CT) synthesis has been proposed as an alternative to physics based and segmentation-based approaches that assign a population-based tissue density value in order to generate an attenuation map. We propose a novel deep fully convolutional neural network that generates synthetic CTs in a recursive manner by gradually reducing the residuals of the previous network, increasing the overall accuracy and generalisability, while keeping the number of trainable parameters within reasonable limits. The model is trained on a database of 20 pre-acquired MRI/CT pairs and a four-fold random bootstrapped validation with a 80:20 split is performed. Quantitative results show that the proposed framework outperforms a state-of-the-art atlas-based approach decreasing the Mean Absolute Error (MAE) from 131HU to 68HU for the synthetic CTs and reducing the PET reconstruction error from 14.3% to 7.2%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge