Pawel Markiewicz
Improved MR to CT synthesis for PET/MR attenuation correction using Imitation Learning
Aug 27, 2019Abstract:The ability to synthesise Computed Tomography images - commonly known as pseudo CT, or pCT - from MRI input data is commonly assessed using an intensity-wise similarity, such as an L2-norm between the ground truth CT and the pCT. However, given that the ultimate purpose is often to use the pCT as an attenuation map ($\mu$-map) in Positron Emission Tomography Magnetic Resonance Imaging (PET/MRI), minimising the error between pCT and CT is not necessarily optimal. The main objective should be to predict a pCT that, when used as $\mu$-map, reconstructs a pseudo PET (pPET) which is as close as possible to the gold standard PET. To this end, we propose a novel multi-hypothesis deep learning framework that generates pCTs by minimising a combination of the pixel-wise error between pCT and CT and a proposed metric-loss that itself is represented by a convolutional neural network (CNN) and aims to minimise subsequent PET residuals. The model is trained on a database of 400 paired MR/CT/PET image slices. Quantitative results show that the network generates pCTs that seem less accurate when evaluating the Mean Absolute Error on the pCT (69.68HU) compared to a baseline CNN (66.25HU), but lead to significant improvement in the PET reconstruction - 115a.u. compared to baseline 140a.u.
Faster PET Reconstruction with Non-Smooth Priors by Randomization and Preconditioning
Sep 21, 2018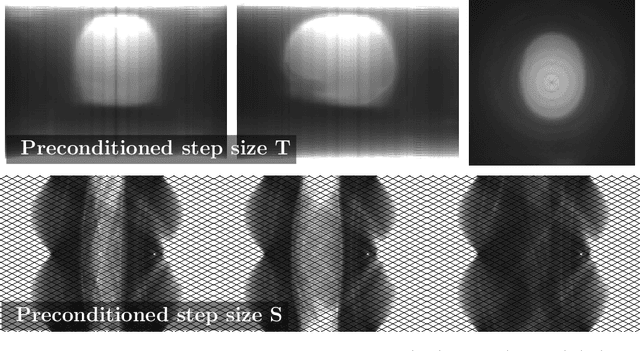
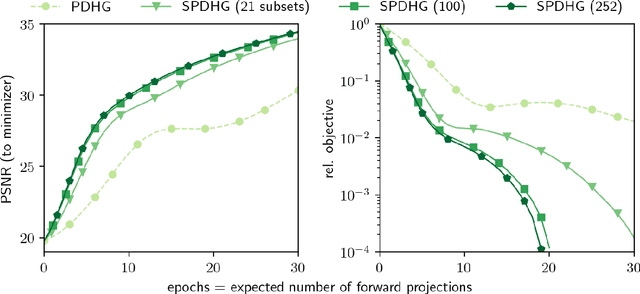
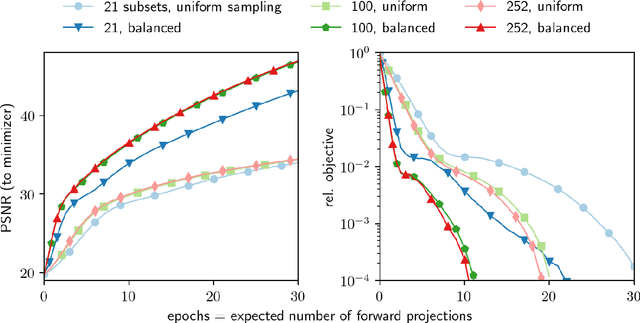
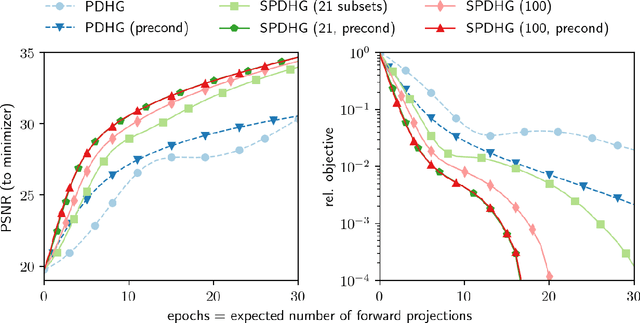
Abstract:Uncompressed clinical data from modern positron emission tomography (PET) scanners are very large, exceeding 350 million data points. The last decades have seen tremendous advancements in mathematical imaging tools many of which lead to non-smooth optimization problems. Most of these tools have not been translated to clinical PET data, as the algorithms for non-smooth problems do not scale well enough for large data sets. In this work, inspired by big data machine learning applications, we use advanced randomized optimization algorithms to solve the PET reconstruction problem for a very large class of non-smooth priors which includes for example total variation, total generalized variation, directional total variation and many constraints. We show on real PET data (FDG and florbetapir) from a Siemens Biograph mMR that a dozen forward (and back) projections are sufficient for a variety of mathematical models to produce clinically relevant images; thus showing that the proposed algorithm is fast enough to bring these models into routine clinical practice. Moreover, the proposed algorithm is as fast on the unregularized problem as the clinical standard OSEM but-in contrast to OSEM-has provable convergence guarantees, robustness and stability for any subset selection.
Deep Boosted Regression for MR to CT Synthesis
Aug 22, 2018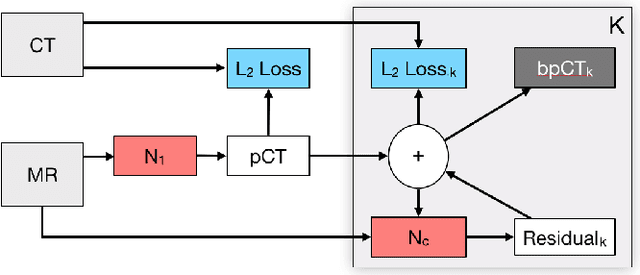
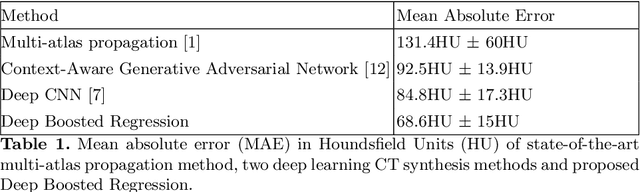
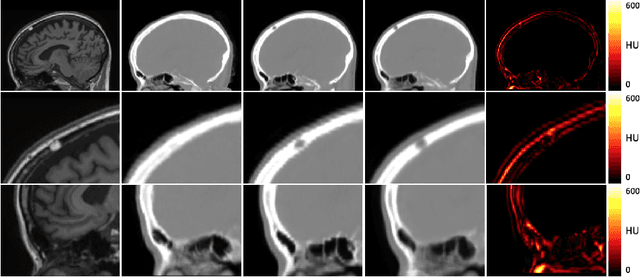
Abstract:Attenuation correction is an essential requirement of positron emission tomography (PET) image reconstruction to allow for accurate quantification. However, attenuation correction is particularly challenging for PET-MRI as neither PET nor magnetic resonance imaging (MRI) can directly image tissue attenuation properties. MRI-based computed tomography (CT) synthesis has been proposed as an alternative to physics based and segmentation-based approaches that assign a population-based tissue density value in order to generate an attenuation map. We propose a novel deep fully convolutional neural network that generates synthetic CTs in a recursive manner by gradually reducing the residuals of the previous network, increasing the overall accuracy and generalisability, while keeping the number of trainable parameters within reasonable limits. The model is trained on a database of 20 pre-acquired MRI/CT pairs and a four-fold random bootstrapped validation with a 80:20 split is performed. Quantitative results show that the proposed framework outperforms a state-of-the-art atlas-based approach decreasing the Mean Absolute Error (MAE) from 131HU to 68HU for the synthetic CTs and reducing the PET reconstruction error from 14.3% to 7.2%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge