Sven Prevrhal
Artificial Intelligence in PET: an Industry Perspective
Jul 14, 2021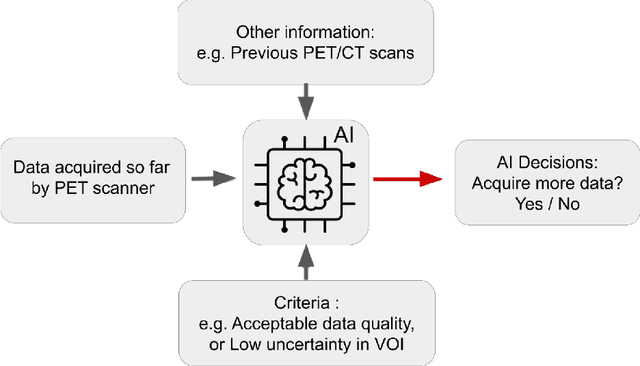
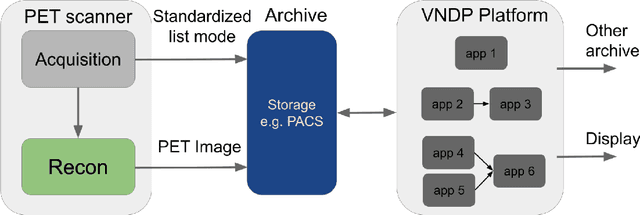
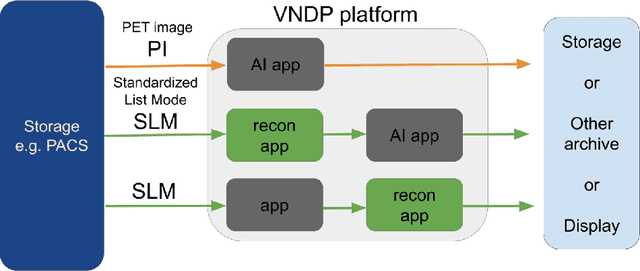
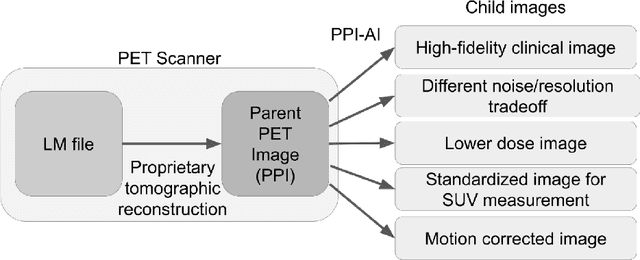
Abstract:Artificial intelligence (AI) has significant potential to positively impact and advance medical imaging, including positron emission tomography (PET) imaging applications. AI has the ability to enhance and optimize all aspects of the PET imaging chain from patient scheduling, patient setup, protocoling, data acquisition, detector signal processing, reconstruction, image processing and interpretation. AI poses industry-specific challenges which will need to be addressed and overcome to maximize the future potentials of AI in PET. This paper provides an overview of these industry-specific challenges for the development, standardization, commercialization, and clinical adoption of AI, and explores the potential enhancements to PET imaging brought on by AI in the near future. In particular, the combination of on-demand image reconstruction, AI, and custom designed data processing workflows may open new possibilities for innovation which would positively impact the industry and ultimately patients.
Improving CCTA based lesions' hemodynamic significance assessment by accounting for partial volume modeling in automatic coronary lumen segmentation
Jun 24, 2019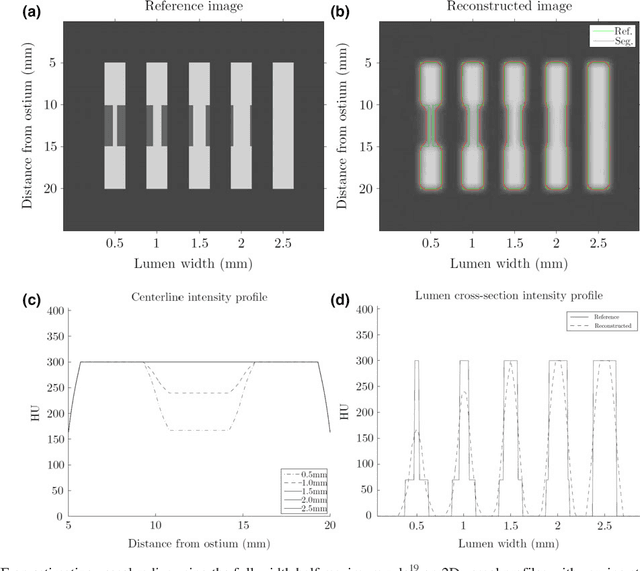
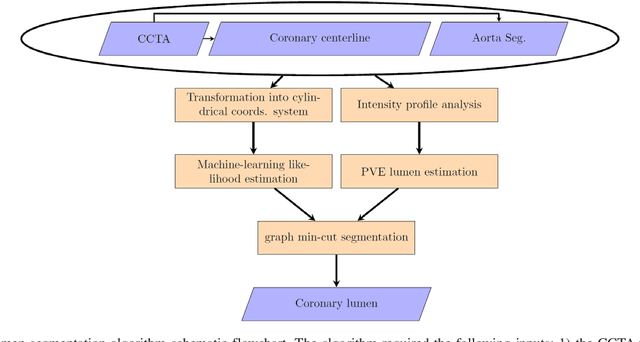
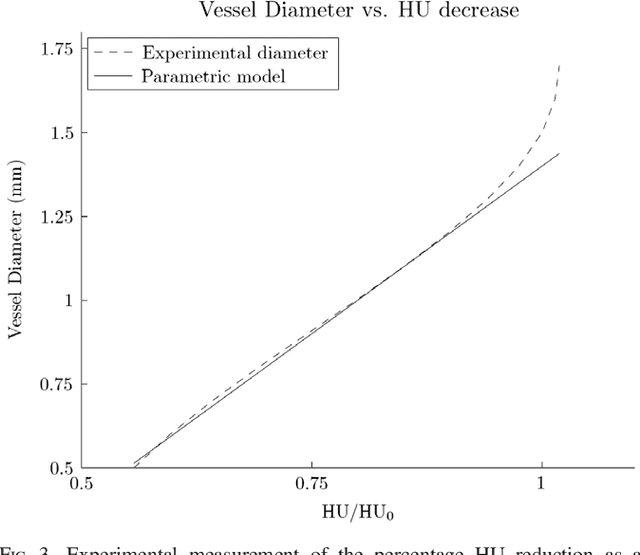
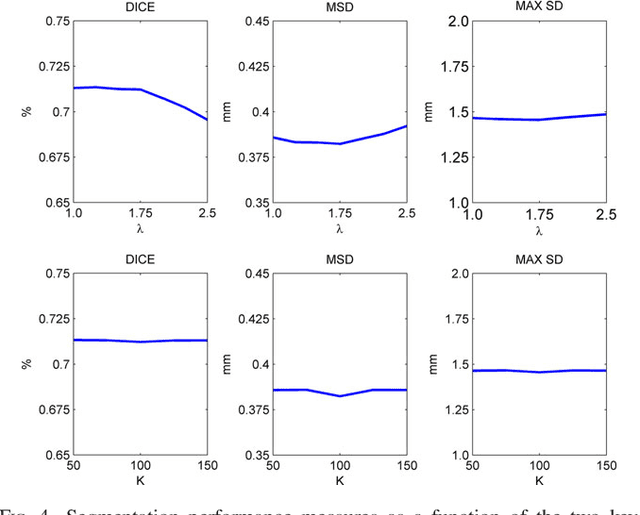
Abstract:Purpose: The goal of this study was to assess the potential added benefit of accounting for partial volume effects (PVE) in an automatic coronary lumen segmentation algorithm from coronary computed tomography angiography (CCTA). Materials and methods: We assessed the potential added value of PVE integration as a part of the automatic coronary lumen segmentation algorithm by means of segmentation accuracy using the MICCAI 2012 challenge framework and by means of flow simulation overall accuracy, sensitivity, specificity, negative and positive predictive values and the receiver operated characteristic (ROC) area under the curve. We also evaluated the potential benefit of accounting for PVE in automatic segmentation for flow-simulation for lesions that were diagnosed as obstructive based on CCTA, which could have indicated a need for an invasive exam and revascularization. Results: Our segmentation algorithm improves the maximal surface distance error by ~39% compared to previously published method on the 18 datasets 50 from the MICCAI 2012 challenge with comparable Dice and mean surface distance. Results with and without accounting for PVE were comparable. In contrast, integrating PVE analysis into an automatic coronary lumen segmentation algorithm improved the flow simulation specificity from 0.6 to 0.68 with the same sensitivity of 0.83. Also, accounting for PVE improved the area under the ROC curve for detecting hemodynamically significant CAD from 0.76 to 0.8 compared to automatic segmentation without PVE analysis with invasive FFR threshold of 0.8 as the reference standard. The improvement in the AUC was statistically significant (N=76, Delong's test, p=0.012). Conclusion: Accounting for the partial volume effects in automatic coronary lumen segmentation algorithms has the potential to improve the accuracy of CCTA-based hemodynamic assessment of coronary artery lesions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge