Mani Vembar
Learning a sparse database for patch-based medical image segmentation
Jun 25, 2019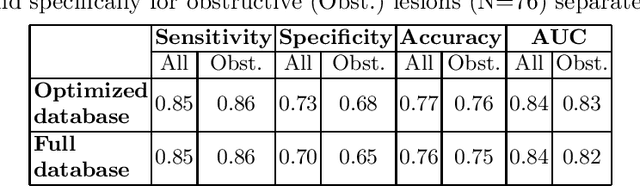
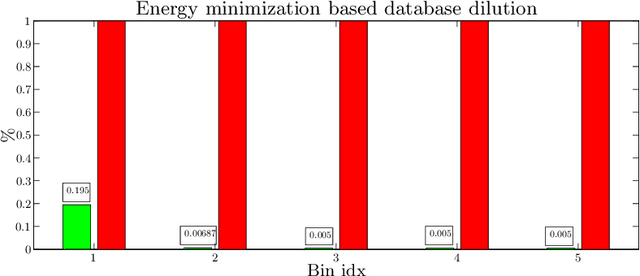
Abstract:We introduce a functional for the learning of an optimal database for patch-based image segmentation with application to coronary lumen segmentation from coronary computed tomography angiography (CCTA) data. The proposed functional consists of fidelity, sparseness and robustness to small-variations terms and their associated weights. Existing work address database optimization by prototype selection aiming to optimize the database by either adding or removing prototypes according to a set of predefined rules. In contrast, we formulate the database optimization task as an energy minimization problem that can be solved using standard numerical tools. We apply the proposed database optimization functional to the task of optimizing a database for patch-base coronary lumen segmentation. Our experiments using the publicly available MICCAI 2012 coronary lumen segmentation challenge data show that optimizing the database using the proposed approach reduced database size by 96% while maintaining the same level of lumen segmentation accuracy. Moreover, we show that the optimized database yields an improved specificity of CCTA based fractional flow reserve (0.73 vs 0.7 for all lesions and 0.68 vs 0.65 for obstructive lesions) using a training set of 132 (76 obstructive) coronary lesions with invasively measured FFR as the reference.
Improving CCTA based lesions' hemodynamic significance assessment by accounting for partial volume modeling in automatic coronary lumen segmentation
Jun 24, 2019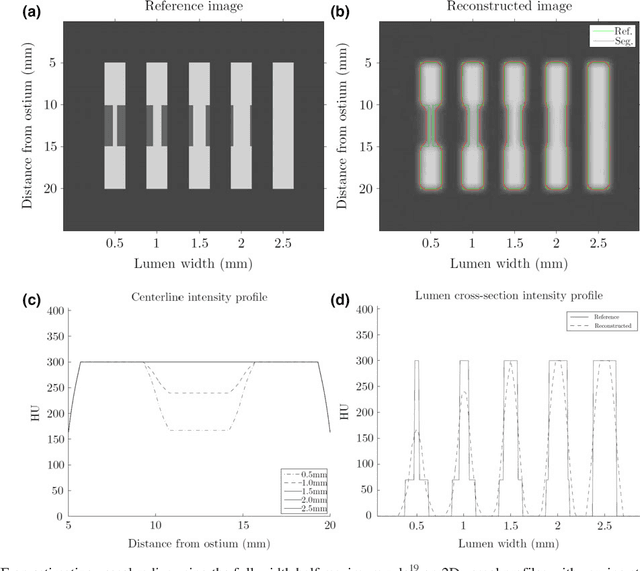
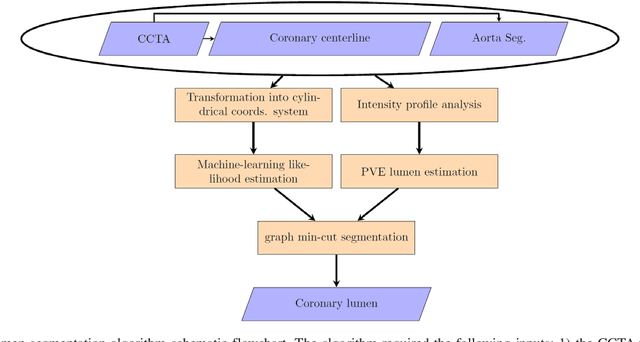
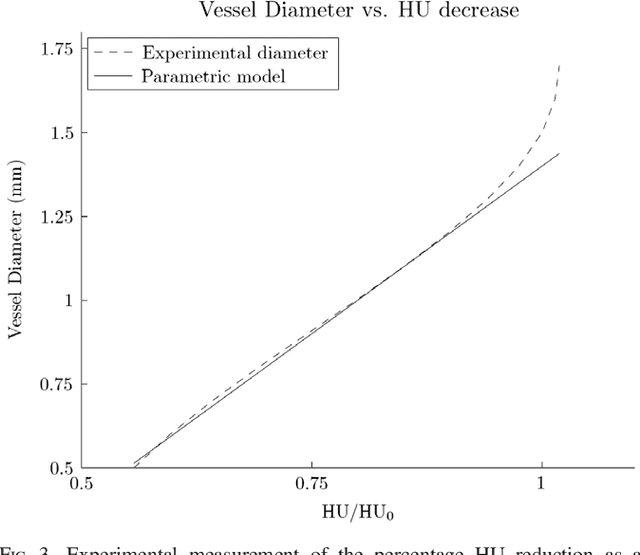
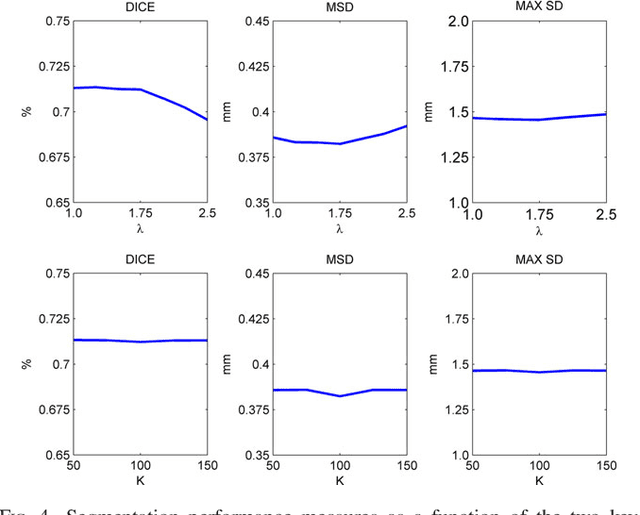
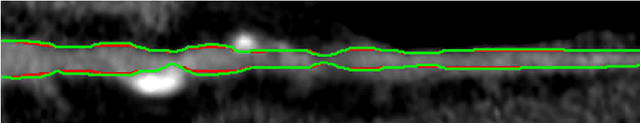
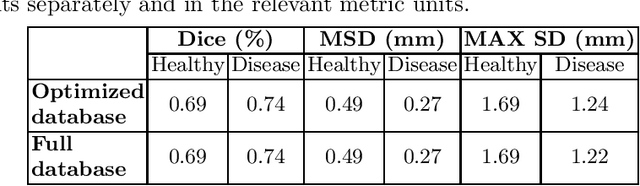
Abstract:Purpose: The goal of this study was to assess the potential added benefit of accounting for partial volume effects (PVE) in an automatic coronary lumen segmentation algorithm from coronary computed tomography angiography (CCTA). Materials and methods: We assessed the potential added value of PVE integration as a part of the automatic coronary lumen segmentation algorithm by means of segmentation accuracy using the MICCAI 2012 challenge framework and by means of flow simulation overall accuracy, sensitivity, specificity, negative and positive predictive values and the receiver operated characteristic (ROC) area under the curve. We also evaluated the potential benefit of accounting for PVE in automatic segmentation for flow-simulation for lesions that were diagnosed as obstructive based on CCTA, which could have indicated a need for an invasive exam and revascularization. Results: Our segmentation algorithm improves the maximal surface distance error by ~39% compared to previously published method on the 18 datasets 50 from the MICCAI 2012 challenge with comparable Dice and mean surface distance. Results with and without accounting for PVE were comparable. In contrast, integrating PVE analysis into an automatic coronary lumen segmentation algorithm improved the flow simulation specificity from 0.6 to 0.68 with the same sensitivity of 0.83. Also, accounting for PVE improved the area under the ROC curve for detecting hemodynamically significant CAD from 0.76 to 0.8 compared to automatic segmentation without PVE analysis with invasive FFR threshold of 0.8 as the reference standard. The improvement in the AUC was statistically significant (N=76, Delong's test, p=0.012). Conclusion: Accounting for the partial volume effects in automatic coronary lumen segmentation algorithms has the potential to improve the accuracy of CCTA-based hemodynamic assessment of coronary artery lesions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge