Dafna Ben Bashat
A multi-centre, multi-device benchmark dataset for landmark-based comprehensive fetal biometry
Dec 19, 2025Abstract:Accurate fetal growth assessment from ultrasound (US) relies on precise biometry measured by manually identifying anatomical landmarks in standard planes. Manual landmarking is time-consuming, operator-dependent, and sensitive to variability across scanners and sites, limiting the reproducibility of automated approaches. There is a need for multi-source annotated datasets to develop artificial intelligence-assisted fetal growth assessment methods. To address this bottleneck, we present an open, multi-centre, multi-device benchmark dataset of fetal US images with expert anatomical landmark annotations for clinically used fetal biometric measurements. These measurements include head bi-parietal and occipito-frontal diameters, abdominal transverse and antero-posterior diameters, and femoral length. The dataset comprises 4,513 de-identified US images from 1,904 subjects acquired at three clinical sites using seven different US devices. We provide standardised, subject-disjoint train/test splits, evaluation code, and baseline results to enable fair and reproducible comparison of methods. Using an automatic biometry model, we quantify domain shift and demonstrate that training and evaluation confined to a single centre substantially overestimate performance relative to multi-centre testing. To the best of our knowledge, this is the first publicly available multi-centre, multi-device, landmark-annotated dataset that covers all primary fetal biometry measures, providing a robust benchmark for domain adaptation and multi-centre generalisation in fetal biometry and enabling more reliable AI-assisted fetal growth assessment across centres. All data, annotations, training code, and evaluation pipelines are made publicly available.
Advances in Automated Fetal Brain MRI Segmentation and Biometry: Insights from the FeTA 2024 Challenge
May 05, 2025



Abstract:Accurate fetal brain tissue segmentation and biometric analysis are essential for studying brain development in utero. The FeTA Challenge 2024 advanced automated fetal brain MRI analysis by introducing biometry prediction as a new task alongside tissue segmentation. For the first time, our diverse multi-centric test set included data from a new low-field (0.55T) MRI dataset. Evaluation metrics were also expanded to include the topology-specific Euler characteristic difference (ED). Sixteen teams submitted segmentation methods, most of which performed consistently across both high- and low-field scans. However, longitudinal trends indicate that segmentation accuracy may be reaching a plateau, with results now approaching inter-rater variability. The ED metric uncovered topological differences that were missed by conventional metrics, while the low-field dataset achieved the highest segmentation scores, highlighting the potential of affordable imaging systems when paired with high-quality reconstruction. Seven teams participated in the biometry task, but most methods failed to outperform a simple baseline that predicted measurements based solely on gestational age, underscoring the challenge of extracting reliable biometric estimates from image data alone. Domain shift analysis identified image quality as the most significant factor affecting model generalization, with super-resolution pipelines also playing a substantial role. Other factors, such as gestational age, pathology, and acquisition site, had smaller, though still measurable, effects. Overall, FeTA 2024 offers a comprehensive benchmark for multi-class segmentation and biometry estimation in fetal brain MRI, underscoring the need for data-centric approaches, improved topological evaluation, and greater dataset diversity to enable clinically robust and generalizable AI tools.
Contour Dice loss for structures with Fuzzy and Complex Boundaries in Fetal MRI
Sep 25, 2022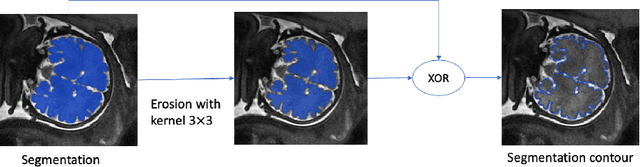
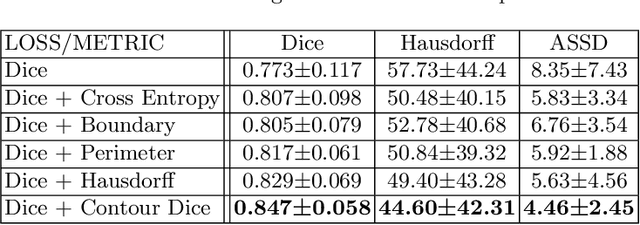
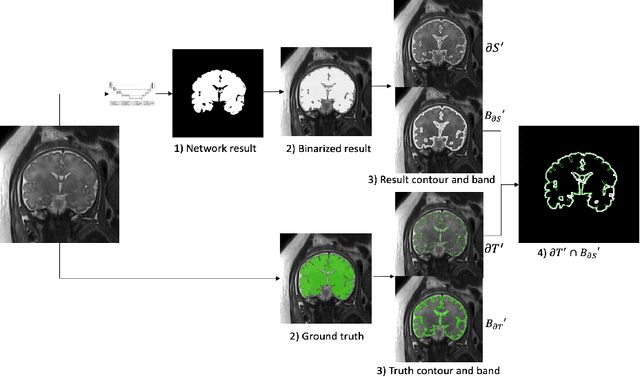
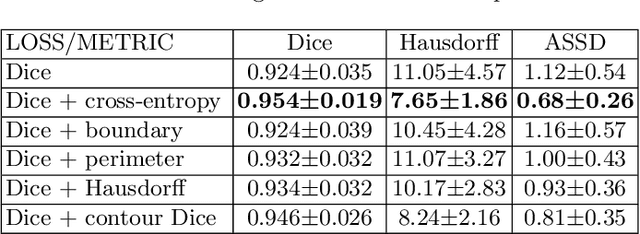
Abstract:Volumetric measurements of fetal structures in MRI are time consuming and error prone and therefore require automatic segmentation. Placenta segmentation and accurate fetal brain segmentation for gyrification assessment are particularly challenging because of the placenta fuzzy boundaries and the fetal brain cortex complex foldings. In this paper, we study the use of the Contour Dice loss for both problems and compare it to other boundary losses and to the combined Dice and Cross-Entropy loss. The loss is computed efficiently for each slice via erosion, dilation and XOR operators. We describe a new formulation of the loss akin to the Contour Dice metric. The combination of the Dice loss and the Contour Dice yielded the best performance for placenta segmentation. For fetal brain segmentation, the best performing loss was the combined Dice with Cross-Entropy loss followed by the Dice with Contour Dice loss, which performed better than other boundary losses.
Partial annotations for the segmentation of large structures with low annotation cost
Sep 25, 2022Abstract:Deep learning methods have been shown to be effective for the automatic segmentation of structures and pathologies in medical imaging. However, they require large annotated datasets, whose manual segmentation is a tedious and time-consuming task, especially for large structures. We present a new method of partial annotations that uses a small set of consecutive annotated slices from each scan with an annotation effort that is equal to that of only few annotated cases. The training with partial annotations is performed by using only annotated blocks, incorporating information about slices outside the structure of interest and modifying a batch loss function to consider only the annotated slices. To facilitate training in a low data regime, we use a two-step optimization process. We tested the method with the popular soft Dice loss for the fetal body segmentation task in two MRI sequences, TRUFI and FIESTA, and compared full annotation regime to partial annotations with a similar annotation effort. For TRUFI data, the use of partial annotations yielded slightly better performance on average compared to full annotations with an increase in Dice score from 0.936 to 0.942, and a substantial decrease in Standard Deviations (STD) of Dice score by 22% and Average Symmetric Surface Distance (ASSD) by 15%. For the FIESTA sequence, partial annotations also yielded a decrease in STD of the Dice score and ASSD metrics by 27.5% and 33% respectively for in-distribution data, and a substantial improvement also in average performance on out-of-distribution data, increasing Dice score from 0.84 to 0.9 and decreasing ASSD from 7.46 to 4.01 mm. The two-step optimization process was helpful for partial annotations for both in-distribution and out-of-distribution data. The partial annotations method with the two-step optimizer is therefore recommended to improve segmentation performance under low data regime.
* 10 pages, 4 figures
Automatic fetal fat quantification from MRI
Sep 08, 2022



Abstract:Normal fetal adipose tissue (AT) development is essential for perinatal well-being. AT, or simply fat, stores energy in the form of lipids. Malnourishment may result in excessive or depleted adiposity. Although previous studies showed a correlation between the amount of AT and perinatal outcome, prenatal assessment of AT is limited by lacking quantitative methods. Using magnetic resonance imaging (MRI), 3D fat- and water-only images of the entire fetus can be obtained from two point Dixon images to enable AT lipid quantification. This paper is the first to present a methodology for developing a deep learning based method for fetal fat segmentation based on Dixon MRI. It optimizes radiologists' manual fetal fat delineation time to produce annotated training dataset. It consists of two steps: 1) model-based semi-automatic fetal fat segmentations, reviewed and corrected by a radiologist; 2) automatic fetal fat segmentation using DL networks trained on the resulting annotated dataset. Three DL networks were trained. We show a significant improvement in segmentation times (3:38 hours to < 1 hour) and observer variability (Dice of 0.738 to 0.906) compared to manual segmentation. Automatic segmentation of 24 test cases with the 3D Residual U-Net, nn-UNet and SWIN-UNetR transformer networks yields a mean Dice score of 0.863, 0.787 and 0.856, respectively. These results are better than the manual observer variability, and comparable to automatic adult and pediatric fat segmentation. A radiologist reviewed and corrected six new independent cases segmented using the best performing network, resulting in a Dice score of 0.961 and a significantly reduced correction time of 15:20 minutes. Using these novel segmentation methods and short MRI acquisition time, whole body subcutaneous lipids can be quantified for individual fetuses in the clinic and large-cohort research.
BiometryNet: Landmark-based Fetal Biometry Estimation from Standard Ultrasound Planes
Jun 29, 2022



Abstract:Fetal growth assessment from ultrasound is based on a few biometric measurements that are performed manually and assessed relative to the expected gestational age. Reliable biometry estimation depends on the precise detection of landmarks in standard ultrasound planes. Manual annotation can be time-consuming and operator dependent task, and may results in high measurements variability. Existing methods for automatic fetal biometry rely on initial automatic fetal structure segmentation followed by geometric landmark detection. However, segmentation annotations are time-consuming and may be inaccurate, and landmark detection requires developing measurement-specific geometric methods. This paper describes BiometryNet, an end-to-end landmark regression framework for fetal biometry estimation that overcomes these limitations. It includes a novel Dynamic Orientation Determination (DOD) method for enforcing measurement-specific orientation consistency during network training. DOD reduces variabilities in network training, increases landmark localization accuracy, thus yields accurate and robust biometric measurements. To validate our method, we assembled a dataset of 3,398 ultrasound images from 1,829 subjects acquired in three clinical sites with seven different ultrasound devices. Comparison and cross-validation of three different biometric measurements on two independent datasets shows that BiometryNet is robust and yields accurate measurements whose errors are lower than the clinically permissible errors, outperforming other existing automated biometry estimation methods. Code is available at https://github.com/netanellavisdris/fetalbiometry.
Fetal Brain Tissue Annotation and Segmentation Challenge Results
Apr 20, 2022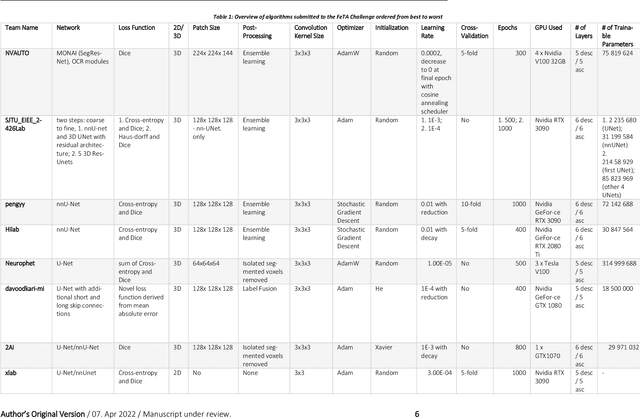
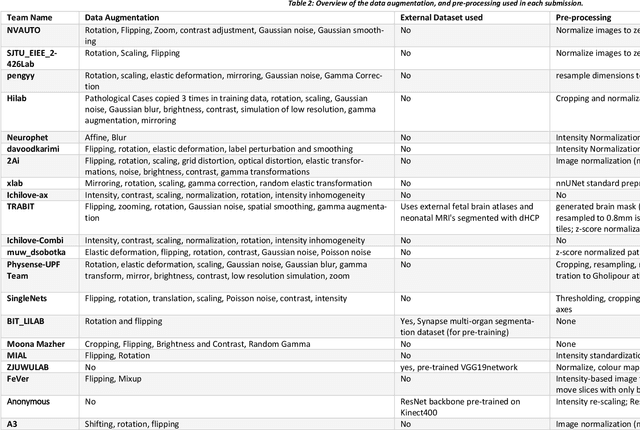
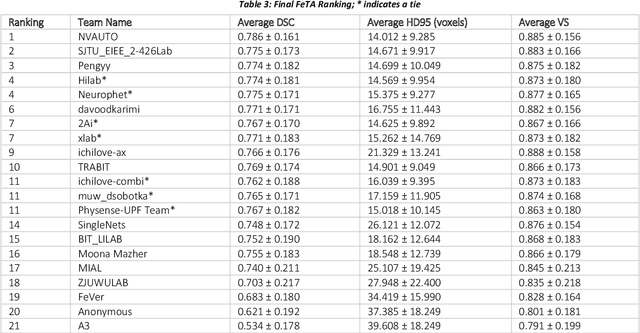
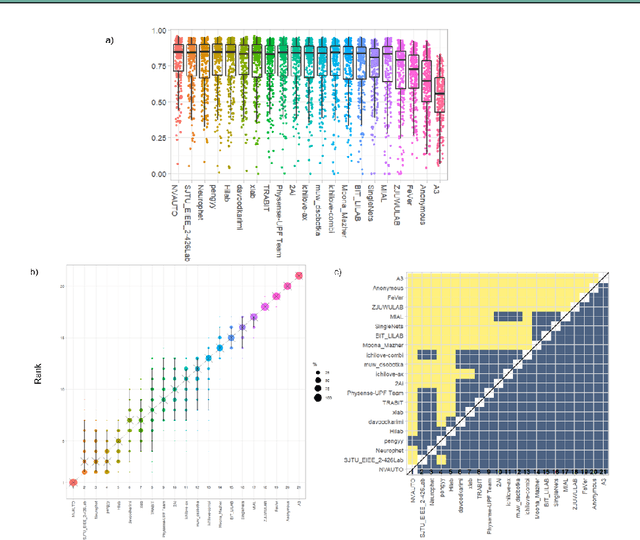
Abstract:In-utero fetal MRI is emerging as an important tool in the diagnosis and analysis of the developing human brain. Automatic segmentation of the developing fetal brain is a vital step in the quantitative analysis of prenatal neurodevelopment both in the research and clinical context. However, manual segmentation of cerebral structures is time-consuming and prone to error and inter-observer variability. Therefore, we organized the Fetal Tissue Annotation (FeTA) Challenge in 2021 in order to encourage the development of automatic segmentation algorithms on an international level. The challenge utilized FeTA Dataset, an open dataset of fetal brain MRI reconstructions segmented into seven different tissues (external cerebrospinal fluid, grey matter, white matter, ventricles, cerebellum, brainstem, deep grey matter). 20 international teams participated in this challenge, submitting a total of 21 algorithms for evaluation. In this paper, we provide a detailed analysis of the results from both a technical and clinical perspective. All participants relied on deep learning methods, mainly U-Nets, with some variability present in the network architecture, optimization, and image pre- and post-processing. The majority of teams used existing medical imaging deep learning frameworks. The main differences between the submissions were the fine tuning done during training, and the specific pre- and post-processing steps performed. The challenge results showed that almost all submissions performed similarly. Four of the top five teams used ensemble learning methods. However, one team's algorithm performed significantly superior to the other submissions, and consisted of an asymmetrical U-Net network architecture. This paper provides a first of its kind benchmark for future automatic multi-tissue segmentation algorithms for the developing human brain in utero.
Automatic linear measurements of the fetal brain on MRI with deep neural networks
Jun 15, 2021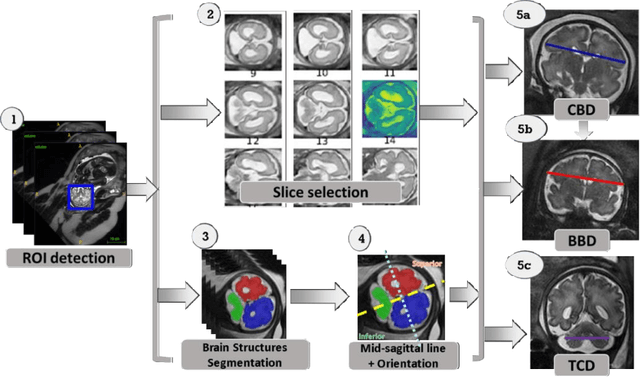
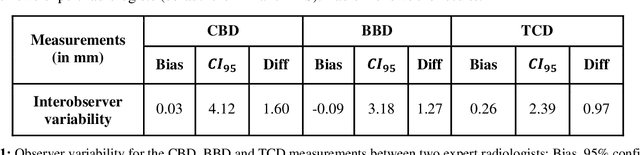
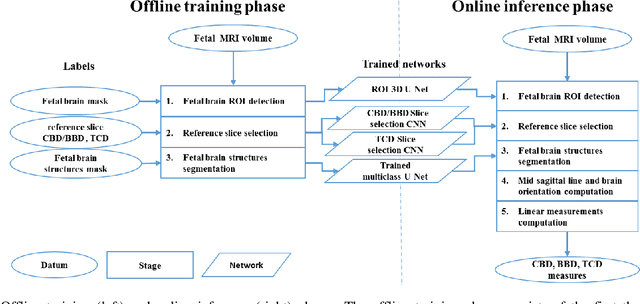
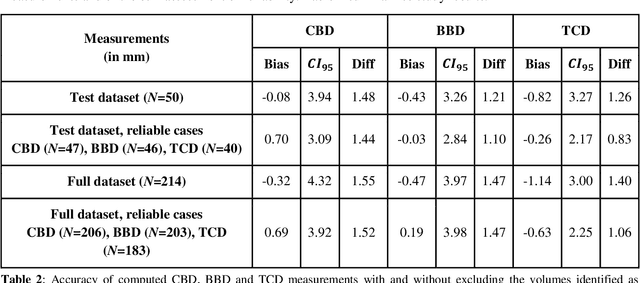
Abstract:Timely, accurate and reliable assessment of fetal brain development is essential to reduce short and long-term risks to fetus and mother. Fetal MRI is increasingly used for fetal brain assessment. Three key biometric linear measurements important for fetal brain evaluation are Cerebral Biparietal Diameter (CBD), Bone Biparietal Diameter (BBD), and Trans-Cerebellum Diameter (TCD), obtained manually by expert radiologists on reference slices, which is time consuming and prone to human error. The aim of this study was to develop a fully automatic method computing the CBD, BBD and TCD measurements from fetal brain MRI. The input is fetal brain MRI volumes which may include the fetal body and the mother's abdomen. The outputs are the measurement values and reference slices on which the measurements were computed. The method, which follows the manual measurements principle, consists of five stages: 1) computation of a Region Of Interest that includes the fetal brain with an anisotropic 3D U-Net classifier; 2) reference slice selection with a Convolutional Neural Network; 3) slice-wise fetal brain structures segmentation with a multiclass U-Net classifier; 4) computation of the fetal brain midsagittal line and fetal brain orientation, and; 5) computation of the measurements. Experimental results on 214 volumes for CBD, BBD and TCD measurements yielded a mean $L_1$ difference of 1.55mm, 1.45mm and 1.23mm respectively, and a Bland-Altman 95% confidence interval ($CI_{95}$) of 3.92mm, 3.98mm and 2.25mm respectively. These results are similar to the manual inter-observer variability. The proposed automatic method for computing biometric linear measurements of the fetal brain from MR imaging achieves human level performance. It has the potential of being a useful method for the assessment of fetal brain biometry in normal and pathological cases, and of improving routine clinical practice.
Compressed sensing for longitudinal MRI: An adaptive-weighted approach
Aug 25, 2017



Abstract:Purpose: Repeated brain MRI scans are performed in many clinical scenarios, such as follow up of patients with tumors and therapy response assessment. In this paper, the authors show an approach to utilize former scans of the patient for the acceleration of repeated MRI scans. Methods: The proposed approach utilizes the possible similarity of the repeated scans in longitudinal MRI studies. Since similarity is not guaranteed, sampling and reconstruction are adjusted during acquisition to match the actual similarity between the scans. The baseline MR scan is utilized both in the sampling stage, via adaptive sampling, and in the reconstruction stage, with weighted reconstruction. In adaptive sampling, k-space sampling locations are optimized during acquisition. Weighted reconstruction uses the locations of the nonzero coefficients in the sparse domains as a prior in the recovery process. The approach was tested on 2D and 3D MRI scans of patients with brain tumors. Results: The longitudinal adaptive CS MRI (LACS-MRI) scheme provides reconstruction quality which outperforms other CS-based approaches for rapid MRI. Examples are shown on patients with brain tumors and demonstrate improved spatial resolution. Compared with data sampled at Nyquist rate, LACS-MRI exhibits Signal-to-Error Ratio (SER) of 24.8dB with undersampling factor of 16.6 in 3D MRI. Conclusions: The authors have presented a novel method for image reconstruction utilizing similarity of scans in longitudinal MRI studies, where possible. The proposed approach can play a major part and significantly reduce scanning time in many applications that consist of disease follow-up and monitoring of longitudinal changes in brain MRI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge