Daphna Link-Sourani
MBSS-T1: Model-Based Self-Supervised Motion Correction for Robust Cardiac T1 Mapping
Aug 21, 2024Abstract:T1 mapping is a valuable quantitative MRI technique for diagnosing diffuse myocardial diseases. Traditional methods, relying on breath-hold sequences and echo triggering, face challenges with patient compliance and arrhythmias, limiting their effectiveness. Image registration can enable motion-robust T1 mapping, but inherent intensity differences between time points pose a challenge. We introduce MBSS-T1, a self-supervised model for motion correction in cardiac T1 mapping, constrained by physical and anatomical principles. The physical constraints ensure expected signal decay behavior, while the anatomical constraints maintain realistic deformations. The unique combination of these constraints ensures accurate T1 mapping along the longitudinal relaxation axis. MBSS-T1 outperformed baseline deep-learning-based image registration approaches in a 5-fold experiment on a public dataset of 210 patients (STONE sequence) and an internal dataset of 19 patients (MOLLI sequence). MBSS-T1 excelled in model fitting quality (R2: 0.974 vs. 0.941, 0.946), anatomical alignment (Dice score: 0.921 vs. 0.984, 0.988), and expert visual quality assessment for the presence of visible motion artifacts (4.33 vs. 3.34, 3.62). MBSS-T1 has the potential to enable motion-robust T1 mapping for a broader range of patients, overcoming challenges such as arrhythmias, and suboptimal compliance, and allowing for free-breathing T1 mapping without requiring large training datasets.
Automatic fetal fat quantification from MRI
Sep 08, 2022



Abstract:Normal fetal adipose tissue (AT) development is essential for perinatal well-being. AT, or simply fat, stores energy in the form of lipids. Malnourishment may result in excessive or depleted adiposity. Although previous studies showed a correlation between the amount of AT and perinatal outcome, prenatal assessment of AT is limited by lacking quantitative methods. Using magnetic resonance imaging (MRI), 3D fat- and water-only images of the entire fetus can be obtained from two point Dixon images to enable AT lipid quantification. This paper is the first to present a methodology for developing a deep learning based method for fetal fat segmentation based on Dixon MRI. It optimizes radiologists' manual fetal fat delineation time to produce annotated training dataset. It consists of two steps: 1) model-based semi-automatic fetal fat segmentations, reviewed and corrected by a radiologist; 2) automatic fetal fat segmentation using DL networks trained on the resulting annotated dataset. Three DL networks were trained. We show a significant improvement in segmentation times (3:38 hours to < 1 hour) and observer variability (Dice of 0.738 to 0.906) compared to manual segmentation. Automatic segmentation of 24 test cases with the 3D Residual U-Net, nn-UNet and SWIN-UNetR transformer networks yields a mean Dice score of 0.863, 0.787 and 0.856, respectively. These results are better than the manual observer variability, and comparable to automatic adult and pediatric fat segmentation. A radiologist reviewed and corrected six new independent cases segmented using the best performing network, resulting in a Dice score of 0.961 and a significantly reduced correction time of 15:20 minutes. Using these novel segmentation methods and short MRI acquisition time, whole body subcutaneous lipids can be quantified for individual fetuses in the clinic and large-cohort research.
Automatic linear measurements of the fetal brain on MRI with deep neural networks
Jun 15, 2021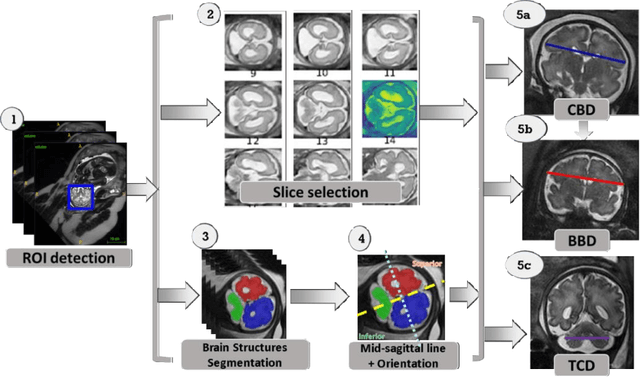
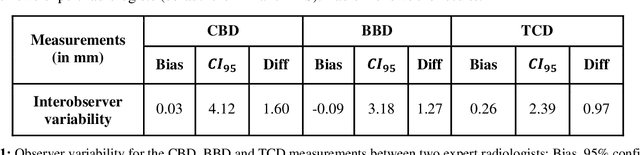
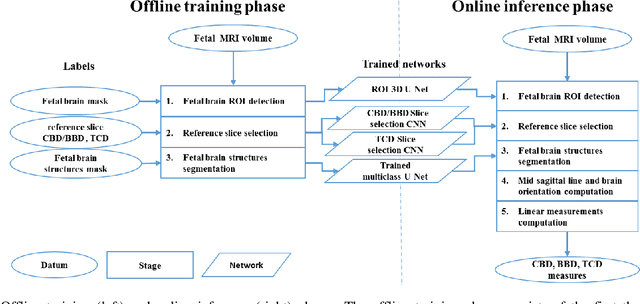
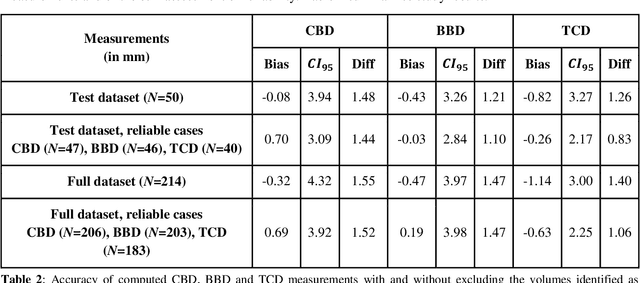
Abstract:Timely, accurate and reliable assessment of fetal brain development is essential to reduce short and long-term risks to fetus and mother. Fetal MRI is increasingly used for fetal brain assessment. Three key biometric linear measurements important for fetal brain evaluation are Cerebral Biparietal Diameter (CBD), Bone Biparietal Diameter (BBD), and Trans-Cerebellum Diameter (TCD), obtained manually by expert radiologists on reference slices, which is time consuming and prone to human error. The aim of this study was to develop a fully automatic method computing the CBD, BBD and TCD measurements from fetal brain MRI. The input is fetal brain MRI volumes which may include the fetal body and the mother's abdomen. The outputs are the measurement values and reference slices on which the measurements were computed. The method, which follows the manual measurements principle, consists of five stages: 1) computation of a Region Of Interest that includes the fetal brain with an anisotropic 3D U-Net classifier; 2) reference slice selection with a Convolutional Neural Network; 3) slice-wise fetal brain structures segmentation with a multiclass U-Net classifier; 4) computation of the fetal brain midsagittal line and fetal brain orientation, and; 5) computation of the measurements. Experimental results on 214 volumes for CBD, BBD and TCD measurements yielded a mean $L_1$ difference of 1.55mm, 1.45mm and 1.23mm respectively, and a Bland-Altman 95% confidence interval ($CI_{95}$) of 3.92mm, 3.98mm and 2.25mm respectively. These results are similar to the manual inter-observer variability. The proposed automatic method for computing biometric linear measurements of the fetal brain from MR imaging achieves human level performance. It has the potential of being a useful method for the assessment of fetal brain biometry in normal and pathological cases, and of improving routine clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge