Walter H. L. Pinaya
Simulating Dynamic Tumor Contrast Enhancement in Breast MRI using Conditional Generative Adversarial Networks
Sep 27, 2024Abstract:This paper presents a method for virtual contrast enhancement in breast MRI, offering a promising non-invasive alternative to traditional contrast agent-based DCE-MRI acquisition. Using a conditional generative adversarial network, we predict DCE-MRI images, including jointly-generated sequences of multiple corresponding DCE-MRI timepoints, from non-contrast-enhanced MRIs, enabling tumor localization and characterization without the associated health risks. Furthermore, we qualitatively and quantitatively evaluate the synthetic DCE-MRI images, proposing a multi-metric Scaled Aggregate Measure (SAMe), assessing their utility in a tumor segmentation downstream task, and conclude with an analysis of the temporal patterns in multi-sequence DCE-MRI generation. Our approach demonstrates promising results in generating realistic and useful DCE-MRI sequences, highlighting the potential of virtual contrast enhancement for improving breast cancer diagnosis and treatment, particularly for patients where contrast agent administration is contraindicated.
Towards Learning Contrast Kinetics with Multi-Condition Latent Diffusion Models
Mar 20, 2024



Abstract:Contrast agents in dynamic contrast enhanced magnetic resonance imaging allow to localize tumors and observe their contrast kinetics, which is essential for cancer characterization and respective treatment decision-making. However, contrast agent administration is not only associated with adverse health risks, but also restricted for patients during pregnancy, and for those with kidney malfunction, or other adverse reactions. With contrast uptake as key biomarker for lesion malignancy, cancer recurrence risk, and treatment response, it becomes pivotal to reduce the dependency on intravenous contrast agent administration. To this end, we propose a multi-conditional latent diffusion model capable of acquisition time-conditioned image synthesis of DCE-MRI temporal sequences. To evaluate medical image synthesis, we additionally propose and validate the Fr\'echet radiomics distance as an image quality measure based on biomarker variability between synthetic and real imaging data. Our results demonstrate our method's ability to generate realistic multi-sequence fat-saturated breast DCE-MRI and uncover the emerging potential of deep learning based contrast kinetics simulation. We publicly share our accessible codebase at https://github.com/RichardObi/ccnet.
Pre- to Post-Contrast Breast MRI Synthesis for Enhanced Tumour Segmentation
Nov 17, 2023Abstract:Despite its benefits for tumour detection and treatment, the administration of contrast agents in dynamic contrast-enhanced MRI (DCE-MRI) is associated with a range of issues, including their invasiveness, bioaccumulation, and a risk of nephrogenic systemic fibrosis. This study explores the feasibility of producing synthetic contrast enhancements by translating pre-contrast T1-weighted fat-saturated breast MRI to their corresponding first DCE-MRI sequence leveraging the capabilities of a generative adversarial network (GAN). Additionally, we introduce a Scaled Aggregate Measure (SAMe) designed for quantitatively evaluating the quality of synthetic data in a principled manner and serving as a basis for selecting the optimal generative model. We assess the generated DCE-MRI data using quantitative image quality metrics and apply them to the downstream task of 3D breast tumour segmentation. Our results highlight the potential of post-contrast DCE-MRI synthesis in enhancing the robustness of breast tumour segmentation models via data augmentation. Our code is available at https://github.com/RichardObi/pre_post_synthesis.
Generative AI for Medical Imaging: extending the MONAI Framework
Jul 27, 2023Abstract:Recent advances in generative AI have brought incredible breakthroughs in several areas, including medical imaging. These generative models have tremendous potential not only to help safely share medical data via synthetic datasets but also to perform an array of diverse applications, such as anomaly detection, image-to-image translation, denoising, and MRI reconstruction. However, due to the complexity of these models, their implementation and reproducibility can be difficult. This complexity can hinder progress, act as a use barrier, and dissuade the comparison of new methods with existing works. In this study, we present MONAI Generative Models, a freely available open-source platform that allows researchers and developers to easily train, evaluate, and deploy generative models and related applications. Our platform reproduces state-of-art studies in a standardised way involving different architectures (such as diffusion models, autoregressive transformers, and GANs), and provides pre-trained models for the community. We have implemented these models in a generalisable fashion, illustrating that their results can be extended to 2D or 3D scenarios, including medical images with different modalities (like CT, MRI, and X-Ray data) and from different anatomical areas. Finally, we adopt a modular and extensible approach, ensuring long-term maintainability and the extension of current applications for future features.
Cross Attention Transformers for Multi-modal Unsupervised Whole-Body PET Anomaly Detection
Apr 14, 2023Abstract:Cancer is a highly heterogeneous condition that can occur almost anywhere in the human body. 18F-fluorodeoxyglucose is an imaging modality commonly used to detect cancer due to its high sensitivity and clear visualisation of the pattern of metabolic activity. Nonetheless, as cancer is highly heterogeneous, it is challenging to train general-purpose discriminative cancer detection models, with data availability and disease complexity often cited as a limiting factor. Unsupervised anomaly detection models have been suggested as a putative solution. These models learn a healthy representation of tissue and detect cancer by predicting deviations from the healthy norm, which requires models capable of accurately learning long-range interactions between organs and their imaging patterns with high levels of expressivity. Such characteristics are suitably satisfied by transformers, which have been shown to generate state-of-the-art results in unsupervised anomaly detection by training on normal data. This work expands upon such approaches by introducing multi-modal conditioning of the transformer via cross-attention i.e. supplying anatomical reference from paired CT. Using 294 whole-body PET/CT samples, we show that our anomaly detection method is robust and capable of achieving accurate cancer localization results even in cases where normal training data is unavailable. In addition, we show the efficacy of this approach on out-of-sample data showcasing the generalizability of this approach with limited training data. Lastly, we propose to combine model uncertainty with a new kernel density estimation approach, and show that it provides clinically and statistically significant improvements when compared to the classic residual-based anomaly maps. Overall, a superior performance is demonstrated against leading state-of-the-art alternatives, drawing attention to the potential of these approaches.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2023:006
Transformer-based normative modelling for anomaly detection of early schizophrenia
Dec 08, 2022Abstract:Despite the impact of psychiatric disorders on clinical health, early-stage diagnosis remains a challenge. Machine learning studies have shown that classifiers tend to be overly narrow in the diagnosis prediction task. The overlap between conditions leads to high heterogeneity among participants that is not adequately captured by classification models. To address this issue, normative approaches have surged as an alternative method. By using a generative model to learn the distribution of healthy brain data patterns, we can identify the presence of pathologies as deviations or outliers from the distribution learned by the model. In particular, deep generative models showed great results as normative models to identify neurological lesions in the brain. However, unlike most neurological lesions, psychiatric disorders present subtle changes widespread in several brain regions, making these alterations challenging to identify. In this work, we evaluate the performance of transformer-based normative models to detect subtle brain changes expressed in adolescents and young adults. We trained our model on 3D MRI scans of neurotypical individuals (N=1,765). Then, we obtained the likelihood of neurotypical controls and psychiatric patients with early-stage schizophrenia from an independent dataset (N=93) from the Human Connectome Project. Using the predicted likelihood of the scans as a proxy for a normative score, we obtained an AUROC of 0.82 when assessing the difference between controls and individuals with early-stage schizophrenia. Our approach surpassed recent normative methods based on brain age and Gaussian Process, showing the promising use of deep generative models to help in individualised analyses.
Denoising Diffusion Models for Out-of-Distribution Detection
Nov 30, 2022Abstract:Out-of-distribution detection is crucial to the safe deployment of machine learning systems. Currently, the state-of-the-art in unsupervised out-of-distribution detection is dominated by generative-based approaches that make use of estimates of the likelihood or other measurements from a generative model. Reconstruction-based methods offer an alternative approach, in which a measure of reconstruction error is used to determine if a sample is out-of-distribution. However, reconstruction-based approaches are less favoured, as they require careful tuning of the model's information bottleneck - such as the size of the latent dimension - to produce good results. In this work, we exploit the view of denoising diffusion probabilistic models (DDPM) as denoising autoencoders where the bottleneck is controlled externally, by means of the amount of noise applied. We propose to use DDPMs to reconstruct an input that has been noised to a range of noise levels, and use the resulting multi-dimensional reconstruction error to classify out-of-distribution inputs. Our approach outperforms not only reconstruction-based methods, but also state-of-the-art generative-based approaches.
Brain Imaging Generation with Latent Diffusion Models
Sep 15, 2022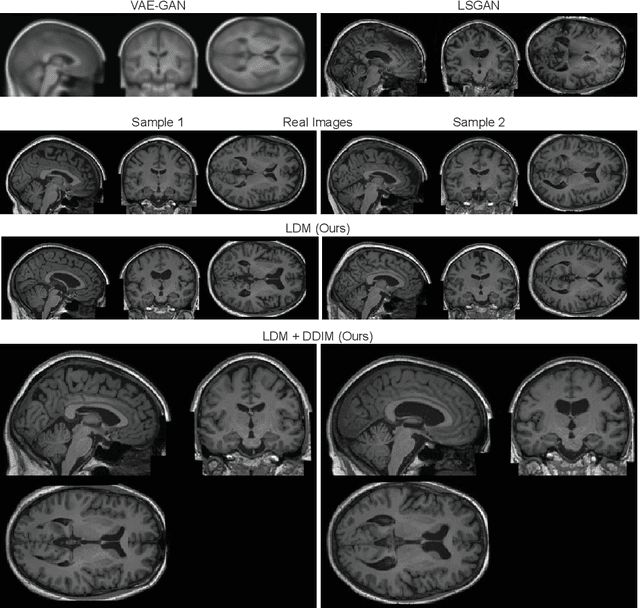
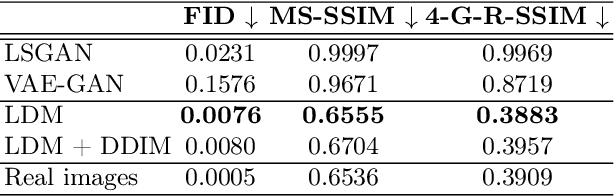
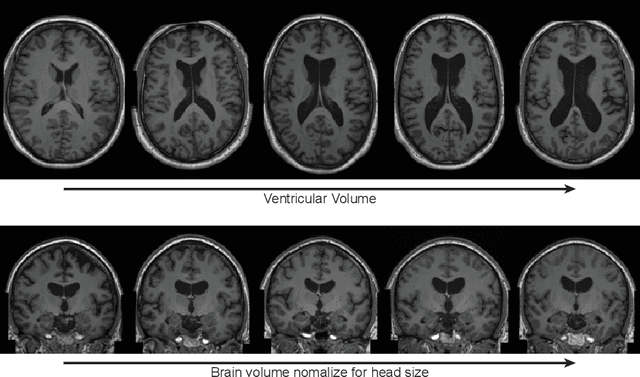
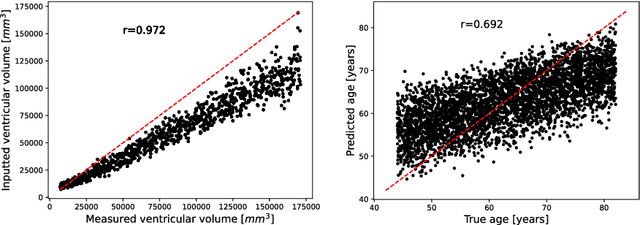
Abstract:Deep neural networks have brought remarkable breakthroughs in medical image analysis. However, due to their data-hungry nature, the modest dataset sizes in medical imaging projects might be hindering their full potential. Generating synthetic data provides a promising alternative, allowing to complement training datasets and conducting medical image research at a larger scale. Diffusion models recently have caught the attention of the computer vision community by producing photorealistic synthetic images. In this study, we explore using Latent Diffusion Models to generate synthetic images from high-resolution 3D brain images. We used T1w MRI images from the UK Biobank dataset (N=31,740) to train our models to learn about the probabilistic distribution of brain images, conditioned on covariables, such as age, sex, and brain structure volumes. We found that our models created realistic data, and we could use the conditioning variables to control the data generation effectively. Besides that, we created a synthetic dataset with 100,000 brain images and made it openly available to the scientific community.
Fast Unsupervised Brain Anomaly Detection and Segmentation with Diffusion Models
Jun 07, 2022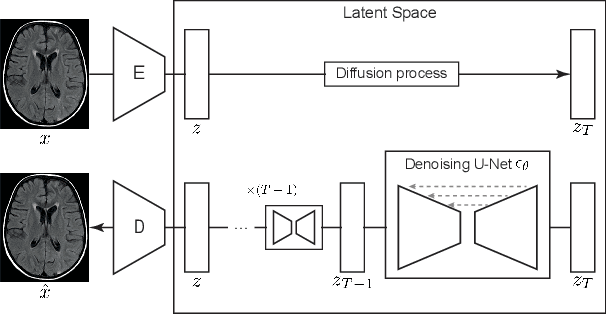
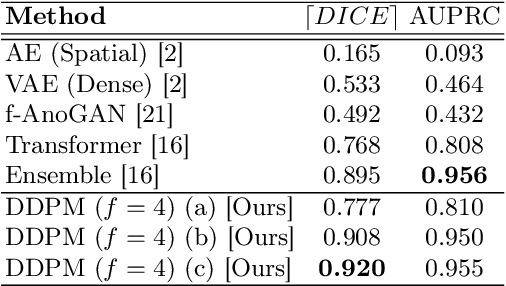
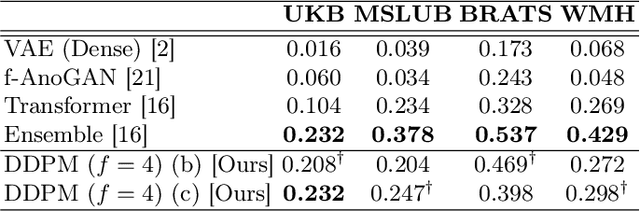
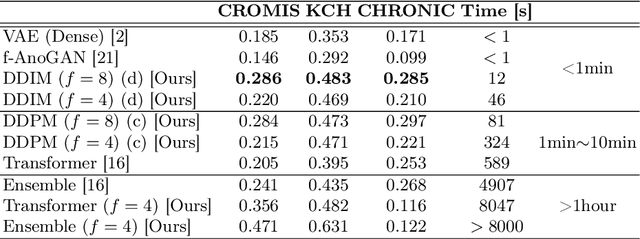
Abstract:Deep generative models have emerged as promising tools for detecting arbitrary anomalies in data, dispensing with the necessity for manual labelling. Recently, autoregressive transformers have achieved state-of-the-art performance for anomaly detection in medical imaging. Nonetheless, these models still have some intrinsic weaknesses, such as requiring images to be modelled as 1D sequences, the accumulation of errors during the sampling process, and the significant inference times associated with transformers. Denoising diffusion probabilistic models are a class of non-autoregressive generative models recently shown to produce excellent samples in computer vision (surpassing Generative Adversarial Networks), and to achieve log-likelihoods that are competitive with transformers while having fast inference times. Diffusion models can be applied to the latent representations learnt by autoencoders, making them easily scalable and great candidates for application to high dimensional data, such as medical images. Here, we propose a method based on diffusion models to detect and segment anomalies in brain imaging. By training the models on healthy data and then exploring its diffusion and reverse steps across its Markov chain, we can identify anomalous areas in the latent space and hence identify anomalies in the pixel space. Our diffusion models achieve competitive performance compared with autoregressive approaches across a series of experiments with 2D CT and MRI data involving synthetic and real pathological lesions with much reduced inference times, making their usage clinically viable.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge