Paul Wright
DIST-CLIP: Arbitrary Metadata and Image Guided MRI Harmonization via Disentangled Anatomy-Contrast Representations
Dec 08, 2025Abstract:Deep learning holds immense promise for transforming medical image analysis, yet its clinical generalization remains profoundly limited. A major barrier is data heterogeneity. This is particularly true in Magnetic Resonance Imaging, where scanner hardware differences, diverse acquisition protocols, and varying sequence parameters introduce substantial domain shifts that obscure underlying biological signals. Data harmonization methods aim to reduce these instrumental and acquisition variability, but existing approaches remain insufficient. When applied to imaging data, image-based harmonization approaches are often restricted by the need for target images, while existing text-guided methods rely on simplistic labels that fail to capture complex acquisition details or are typically restricted to datasets with limited variability, failing to capture the heterogeneity of real-world clinical environments. To address these limitations, we propose DIST-CLIP (Disentangled Style Transfer with CLIP Guidance), a unified framework for MRI harmonization that flexibly uses either target images or DICOM metadata for guidance. Our framework explicitly disentangles anatomical content from image contrast, with the contrast representations being extracted using pre-trained CLIP encoders. These contrast embeddings are then integrated into the anatomical content via a novel Adaptive Style Transfer module. We trained and evaluated DIST-CLIP on diverse real-world clinical datasets, and showed significant improvements in performance when compared against state-of-the-art methods in both style translation fidelity and anatomical preservation, offering a flexible solution for style transfer and standardizing MRI data. Our code and weights will be made publicly available upon publication.
Generalizable automated ischaemic stroke lesion segmentation with vision transformers
Feb 10, 2025
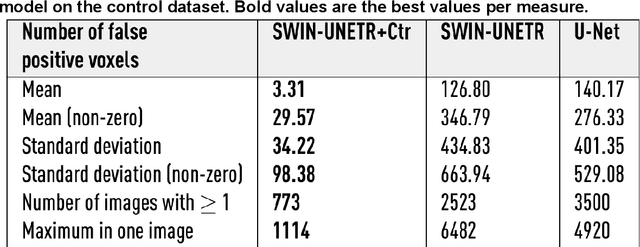
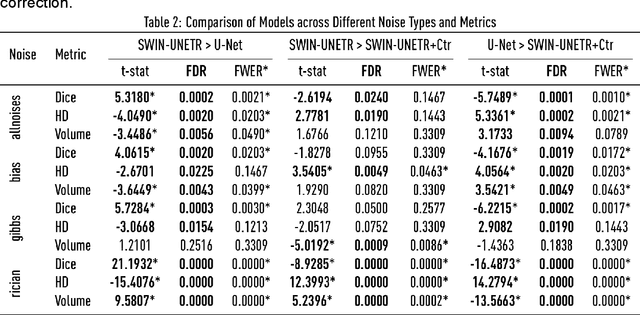
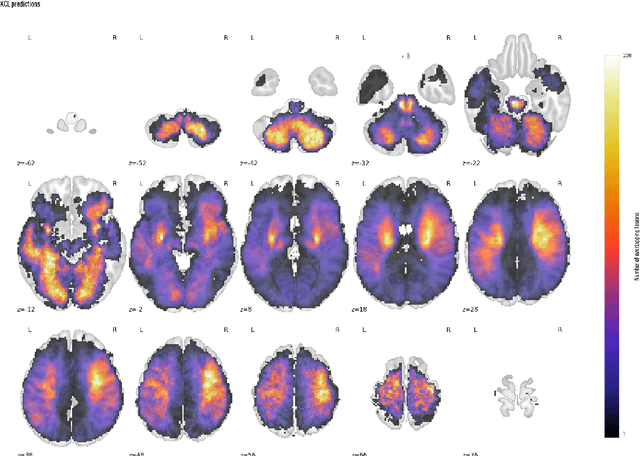
Abstract:Ischaemic stroke, a leading cause of death and disability, critically relies on neuroimaging for characterising the anatomical pattern of injury. Diffusion-weighted imaging (DWI) provides the highest expressivity in ischemic stroke but poses substantial challenges for automated lesion segmentation: susceptibility artefacts, morphological heterogeneity, age-related comorbidities, time-dependent signal dynamics, instrumental variability, and limited labelled data. Current U-Net-based models therefore underperform, a problem accentuated by inadequate evaluation metrics that focus on mean performance, neglecting anatomical, subpopulation, and acquisition-dependent variability. Here, we present a high-performance DWI lesion segmentation tool addressing these challenges through optimized vision transformer-based architectures, integration of 3563 annotated lesions from multi-site data, and algorithmic enhancements, achieving state-of-the-art results. We further propose a novel evaluative framework assessing model fidelity, equity (across demographics and lesion subtypes), anatomical precision, and robustness to instrumental variability, promoting clinical and research utility. This work advances stroke imaging by reconciling model expressivity with domain-specific challenges and redefining performance benchmarks to prioritize equity and generalizability, critical for personalized medicine and mechanistic research.
Deep generative computed perfusion-deficit mapping of ischaemic stroke
Feb 03, 2025



Abstract:Focal deficits in ischaemic stroke result from impaired perfusion downstream of a critical vascular occlusion. While parenchymal lesions are traditionally used to predict clinical deficits, the underlying pattern of disrupted perfusion provides information upstream of the lesion, potentially yielding earlier predictive and localizing signals. Such perfusion maps can be derived from routine CT angiography (CTA) widely deployed in clinical practice. Analysing computed perfusion maps from 1,393 CTA-imaged-patients with acute ischaemic stroke, we use deep generative inference to localise neural substrates of NIHSS sub-scores. We show that our approach replicates known lesion-deficit relations without knowledge of the lesion itself and reveals novel neural dependents. The high achieved anatomical fidelity suggests acute CTA-derived computed perfusion maps may be of substantial clinical-and-scientific value in rich phenotyping of acute stroke. Using only hyperacute imaging, deep generative inference could power highly expressive models of functional anatomical relations in ischaemic stroke within the pre-interventional window.
Framework to generate perfusion map from CT and CTA images in patients with acute ischemic stroke: A longitudinal and cross-sectional study
Apr 05, 2024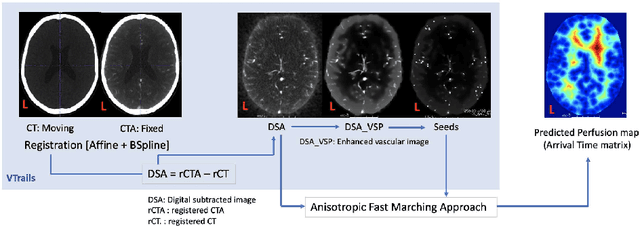
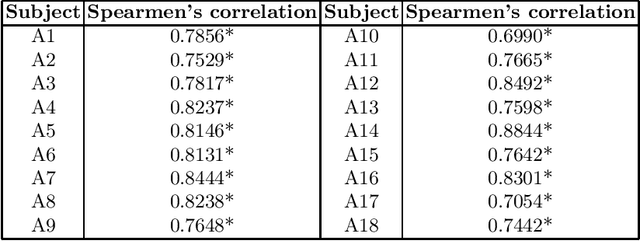
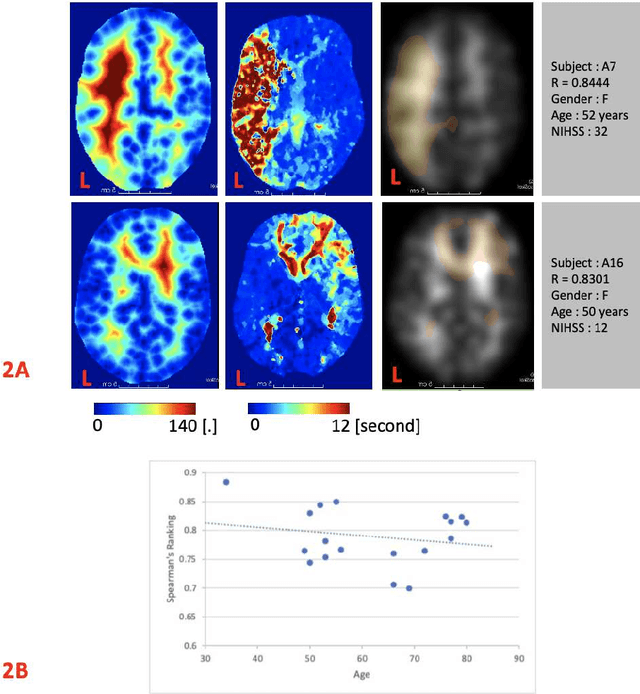
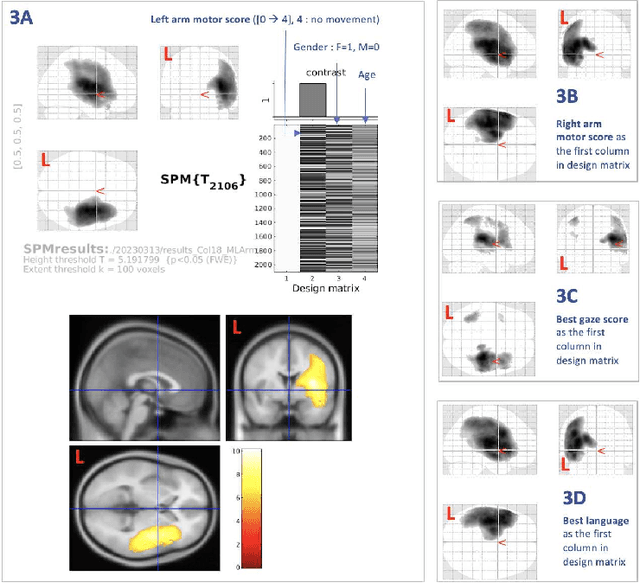
Abstract:Stroke is a leading cause of disability and death. Effective treatment decisions require early and informative vascular imaging. 4D perfusion imaging is ideal but rarely available within the first hour after stroke, whereas plain CT and CTA usually are. Hence, we propose a framework to extract a predicted perfusion map (PPM) derived from CT and CTA images. In all eighteen patients, we found significantly high spatial similarity (with average Spearman's correlation = 0.7893) between our predicted perfusion map (PPM) and the T-max map derived from 4D-CTP. Voxelwise correlations between the PPM and National Institutes of Health Stroke Scale (NIHSS) subscores for L/R hand motor, gaze, and language on a large cohort of 2,110 subjects reliably mapped symptoms to expected infarct locations. Therefore our PPM could serve as an alternative for 4D perfusion imaging, if the latter is unavailable, to investigate blood perfusion in the first hours after hospital admission.
Unsupervised 3D out-of-distribution detection with latent diffusion models
Jul 07, 2023


Abstract:Methods for out-of-distribution (OOD) detection that scale to 3D data are crucial components of any real-world clinical deep learning system. Classic denoising diffusion probabilistic models (DDPMs) have been recently proposed as a robust way to perform reconstruction-based OOD detection on 2D datasets, but do not trivially scale to 3D data. In this work, we propose to use Latent Diffusion Models (LDMs), which enable the scaling of DDPMs to high-resolution 3D medical data. We validate the proposed approach on near- and far-OOD datasets and compare it to a recently proposed, 3D-enabled approach using Latent Transformer Models (LTMs). Not only does the proposed LDM-based approach achieve statistically significant better performance, it also shows less sensitivity to the underlying latent representation, more favourable memory scaling, and produces better spatial anomaly maps. Code is available at https://github.com/marksgraham/ddpm-ood
Fast Unsupervised Brain Anomaly Detection and Segmentation with Diffusion Models
Jun 07, 2022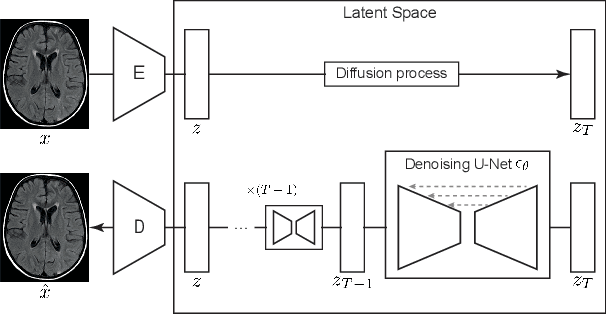
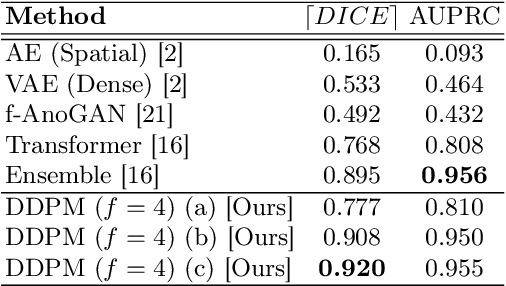
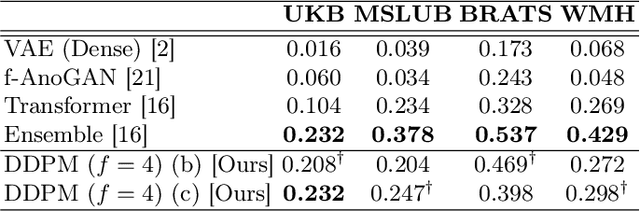
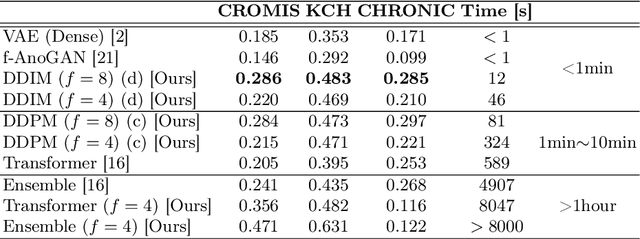
Abstract:Deep generative models have emerged as promising tools for detecting arbitrary anomalies in data, dispensing with the necessity for manual labelling. Recently, autoregressive transformers have achieved state-of-the-art performance for anomaly detection in medical imaging. Nonetheless, these models still have some intrinsic weaknesses, such as requiring images to be modelled as 1D sequences, the accumulation of errors during the sampling process, and the significant inference times associated with transformers. Denoising diffusion probabilistic models are a class of non-autoregressive generative models recently shown to produce excellent samples in computer vision (surpassing Generative Adversarial Networks), and to achieve log-likelihoods that are competitive with transformers while having fast inference times. Diffusion models can be applied to the latent representations learnt by autoencoders, making them easily scalable and great candidates for application to high dimensional data, such as medical images. Here, we propose a method based on diffusion models to detect and segment anomalies in brain imaging. By training the models on healthy data and then exploring its diffusion and reverse steps across its Markov chain, we can identify anomalous areas in the latent space and hence identify anomalies in the pixel space. Our diffusion models achieve competitive performance compared with autoregressive approaches across a series of experiments with 2D CT and MRI data involving synthetic and real pathological lesions with much reduced inference times, making their usage clinically viable.
Transformer-based out-of-distribution detection for clinically safe segmentation
May 21, 2022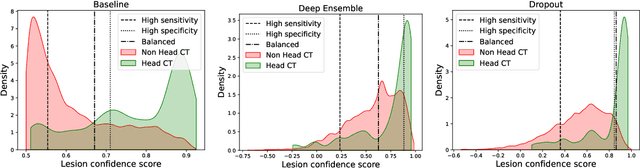
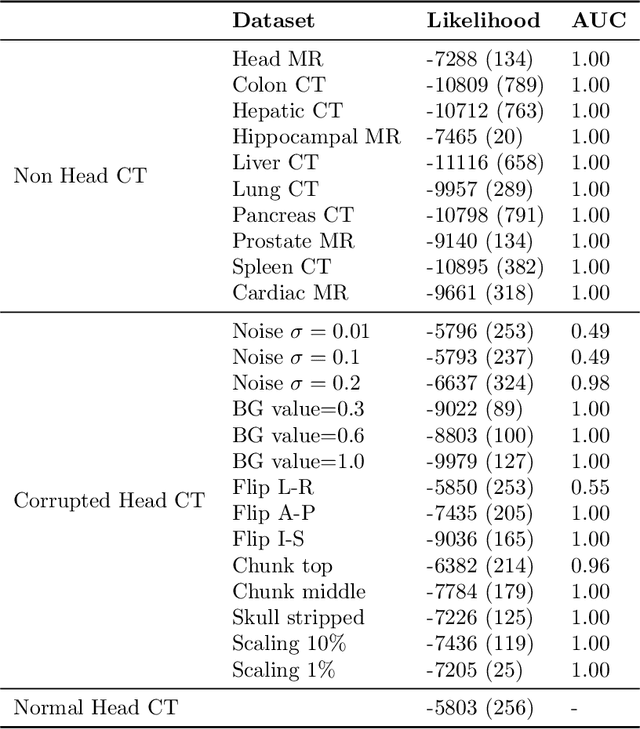
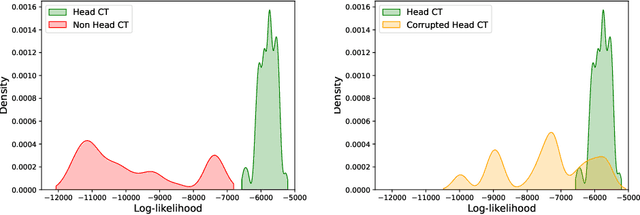
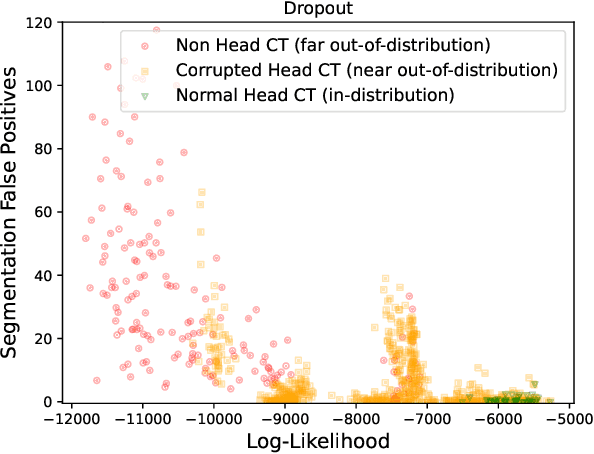
Abstract:In a clinical setting it is essential that deployed image processing systems are robust to the full range of inputs they might encounter and, in particular, do not make confidently wrong predictions. The most popular approach to safe processing is to train networks that can provide a measure of their uncertainty, but these tend to fail for inputs that are far outside the training data distribution. Recently, generative modelling approaches have been proposed as an alternative; these can quantify the likelihood of a data sample explicitly, filtering out any out-of-distribution (OOD) samples before further processing is performed. In this work, we focus on image segmentation and evaluate several approaches to network uncertainty in the far-OOD and near-OOD cases for the task of segmenting haemorrhages in head CTs. We find all of these approaches are unsuitable for safe segmentation as they provide confidently wrong predictions when operating OOD. We propose performing full 3D OOD detection using a VQ-GAN to provide a compressed latent representation of the image and a transformer to estimate the data likelihood. Our approach successfully identifies images in both the far- and near-OOD cases. We find a strong relationship between image likelihood and the quality of a model's segmentation, making this approach viable for filtering images unsuitable for segmentation. To our knowledge, this is the first time transformers have been applied to perform OOD detection on 3D image data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge