Pedro F Da Costa
Transformer-based normative modelling for anomaly detection of early schizophrenia
Dec 08, 2022Abstract:Despite the impact of psychiatric disorders on clinical health, early-stage diagnosis remains a challenge. Machine learning studies have shown that classifiers tend to be overly narrow in the diagnosis prediction task. The overlap between conditions leads to high heterogeneity among participants that is not adequately captured by classification models. To address this issue, normative approaches have surged as an alternative method. By using a generative model to learn the distribution of healthy brain data patterns, we can identify the presence of pathologies as deviations or outliers from the distribution learned by the model. In particular, deep generative models showed great results as normative models to identify neurological lesions in the brain. However, unlike most neurological lesions, psychiatric disorders present subtle changes widespread in several brain regions, making these alterations challenging to identify. In this work, we evaluate the performance of transformer-based normative models to detect subtle brain changes expressed in adolescents and young adults. We trained our model on 3D MRI scans of neurotypical individuals (N=1,765). Then, we obtained the likelihood of neurotypical controls and psychiatric patients with early-stage schizophrenia from an independent dataset (N=93) from the Human Connectome Project. Using the predicted likelihood of the scans as a proxy for a normative score, we obtained an AUROC of 0.82 when assessing the difference between controls and individuals with early-stage schizophrenia. Our approach surpassed recent normative methods based on brain age and Gaussian Process, showing the promising use of deep generative models to help in individualised analyses.
Fast Unsupervised Brain Anomaly Detection and Segmentation with Diffusion Models
Jun 07, 2022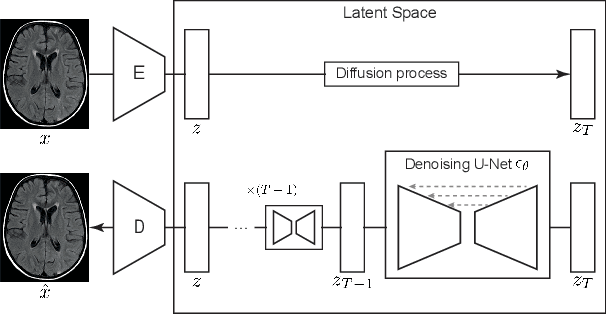
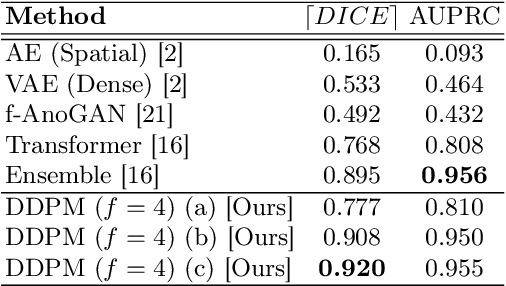
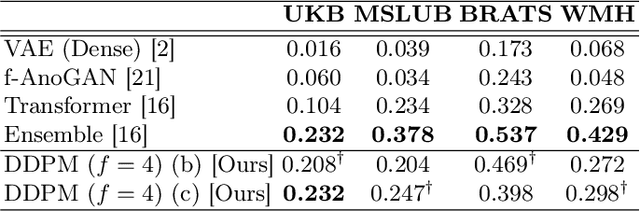
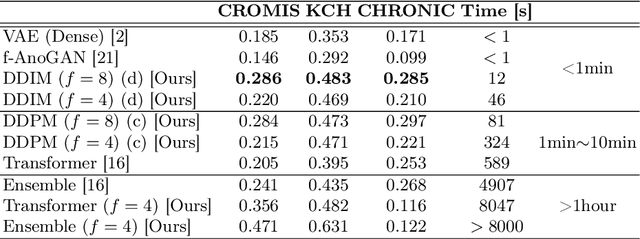
Abstract:Deep generative models have emerged as promising tools for detecting arbitrary anomalies in data, dispensing with the necessity for manual labelling. Recently, autoregressive transformers have achieved state-of-the-art performance for anomaly detection in medical imaging. Nonetheless, these models still have some intrinsic weaknesses, such as requiring images to be modelled as 1D sequences, the accumulation of errors during the sampling process, and the significant inference times associated with transformers. Denoising diffusion probabilistic models are a class of non-autoregressive generative models recently shown to produce excellent samples in computer vision (surpassing Generative Adversarial Networks), and to achieve log-likelihoods that are competitive with transformers while having fast inference times. Diffusion models can be applied to the latent representations learnt by autoencoders, making them easily scalable and great candidates for application to high dimensional data, such as medical images. Here, we propose a method based on diffusion models to detect and segment anomalies in brain imaging. By training the models on healthy data and then exploring its diffusion and reverse steps across its Markov chain, we can identify anomalous areas in the latent space and hence identify anomalies in the pixel space. Our diffusion models achieve competitive performance compared with autoregressive approaches across a series of experiments with 2D CT and MRI data involving synthetic and real pathological lesions with much reduced inference times, making their usage clinically viable.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge