Tom Wong
Learning to Predict Global Atrial Fibrillation Dynamics from Sparse Measurements
Feb 13, 2025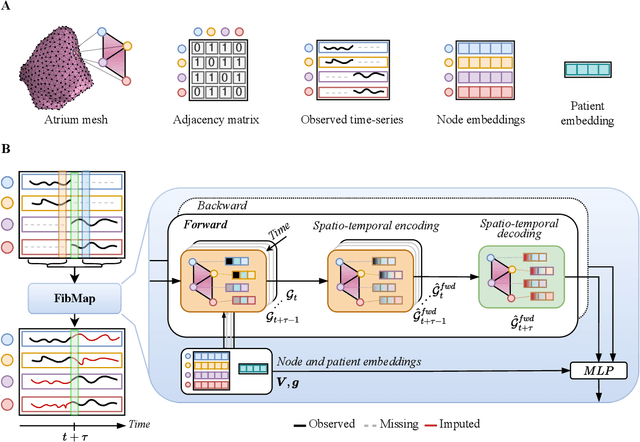
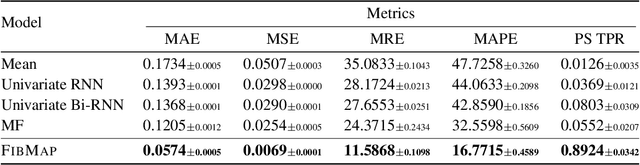
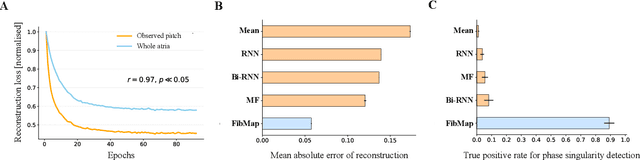
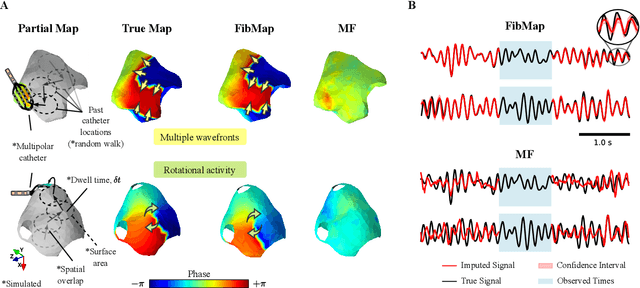
Abstract:Catheter ablation of Atrial Fibrillation (AF) consists of a one-size-fits-all treatment with limited success in persistent AF. This may be due to our inability to map the dynamics of AF with the limited resolution and coverage provided by sequential contact mapping catheters, preventing effective patient phenotyping for personalised, targeted ablation. Here we introduce FibMap, a graph recurrent neural network model that reconstructs global AF dynamics from sparse measurements. Trained and validated on 51 non-contact whole atria recordings, FibMap reconstructs whole atria dynamics from 10% surface coverage, achieving a 210% lower mean absolute error and an order of magnitude higher performance in tracking phase singularities compared to baseline methods. Clinical utility of FibMap is demonstrated on real-world contact mapping recordings, achieving reconstruction fidelity comparable to non-contact mapping. FibMap's state-spaces and patient-specific parameters offer insights for electrophenotyping AF. Integrating FibMap into clinical practice could enable personalised AF care and improve outcomes.
Adaptive Hierarchical Dual Consistency for Semi-Supervised Left Atrium Segmentation on Cross-Domain Data
Sep 20, 2021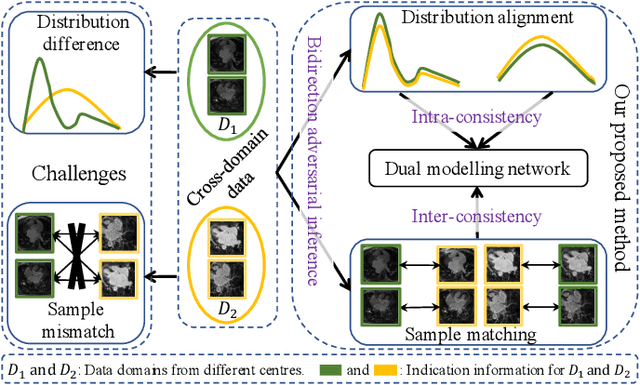
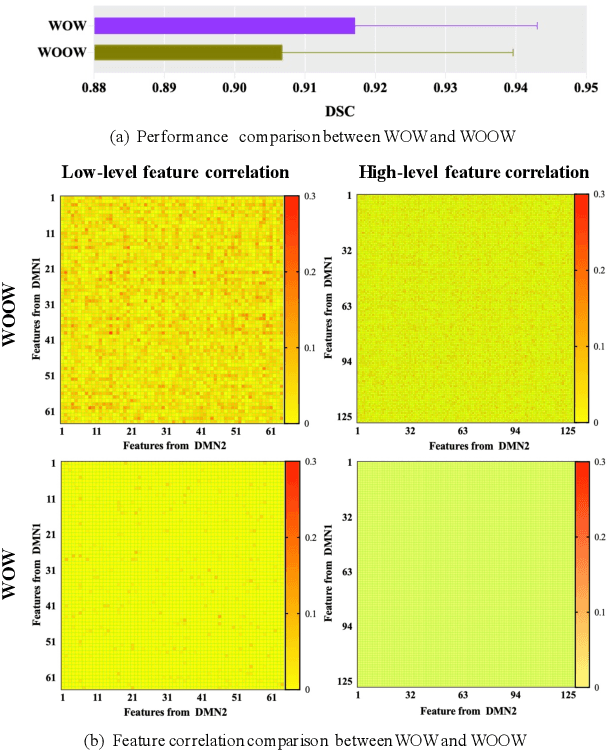
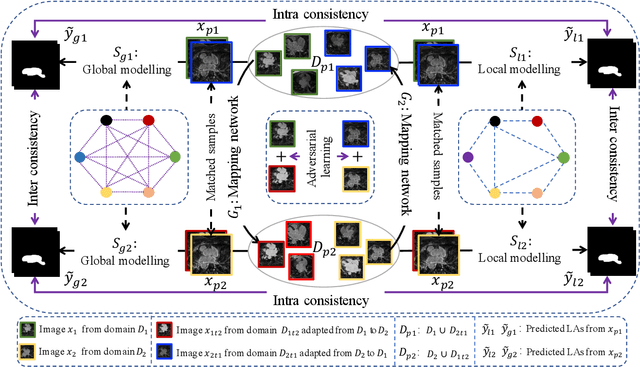
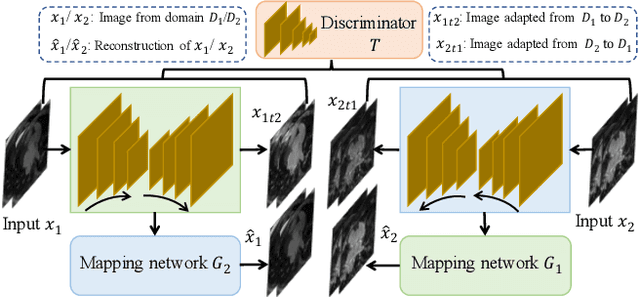
Abstract:Semi-supervised learning provides great significance in left atrium (LA) segmentation model learning with insufficient labelled data. Generalising semi-supervised learning to cross-domain data is of high importance to further improve model robustness. However, the widely existing distribution difference and sample mismatch between different data domains hinder the generalisation of semi-supervised learning. In this study, we alleviate these problems by proposing an Adaptive Hierarchical Dual Consistency (AHDC) for the semi-supervised LA segmentation on cross-domain data. The AHDC mainly consists of a Bidirectional Adversarial Inference module (BAI) and a Hierarchical Dual Consistency learning module (HDC). The BAI overcomes the difference of distributions and the sample mismatch between two different domains. It mainly learns two mapping networks adversarially to obtain two matched domains through mutual adaptation. The HDC investigates a hierarchical dual learning paradigm for cross-domain semi-supervised segmentation based on the obtained matched domains. It mainly builds two dual-modelling networks for mining the complementary information in both intra-domain and inter-domain. For the intra-domain learning, a consistency constraint is applied to the dual-modelling targets to exploit the complementary modelling information. For the inter-domain learning, a consistency constraint is applied to the LAs modelled by two dual-modelling networks to exploit the complementary knowledge among different data domains. We demonstrated the performance of our proposed AHDC on four 3D late gadolinium enhancement cardiac MR (LGE-CMR) datasets from different centres and a 3D CT dataset. Compared to other state-of-the-art methods, our proposed AHDC achieved higher segmentation accuracy, which indicated its capability in the cross-domain semi-supervised LA segmentation.
JAS-GAN: Generative Adversarial Network Based Joint Atrium and Scar Segmentations on Unbalanced Atrial Targets
May 01, 2021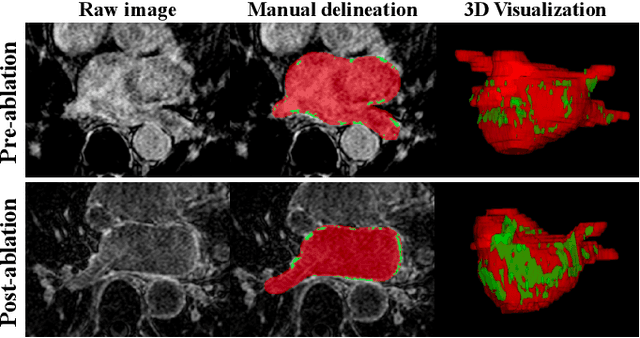
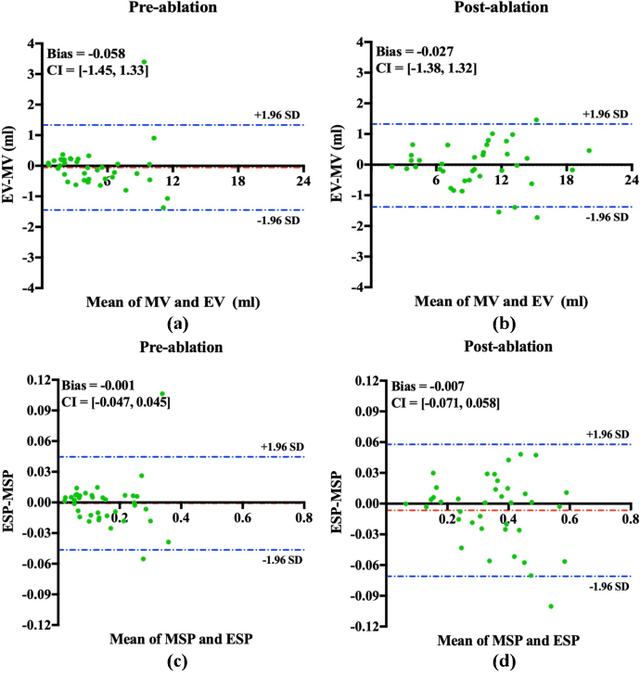
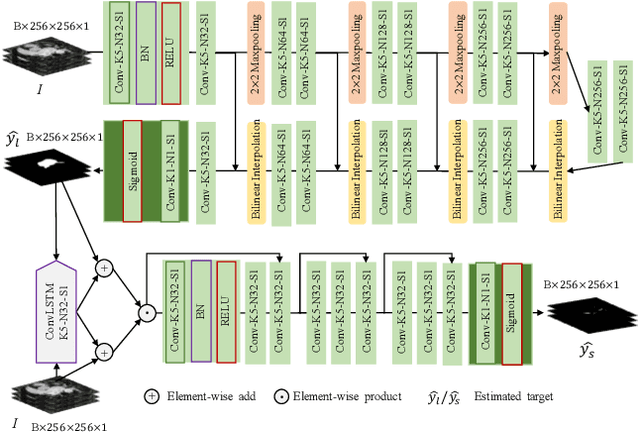
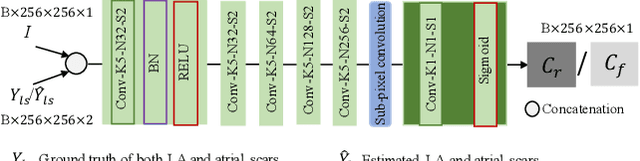
Abstract:Automated and accurate segmentations of left atrium (LA) and atrial scars from late gadolinium-enhanced cardiac magnetic resonance (LGE CMR) images are in high demand for quantifying atrial scars. The previous quantification of atrial scars relies on a two-phase segmentation for LA and atrial scars due to their large volume difference (unbalanced atrial targets). In this paper, we propose an inter-cascade generative adversarial network, namely JAS-GAN, to segment the unbalanced atrial targets from LGE CMR images automatically and accurately in an end-to-end way. Firstly, JAS-GAN investigates an adaptive attention cascade to automatically correlate the segmentation tasks of the unbalanced atrial targets. The adaptive attention cascade mainly models the inclusion relationship of the two unbalanced atrial targets, where the estimated LA acts as the attention map to adaptively focus on the small atrial scars roughly. Then, an adversarial regularization is applied to the segmentation tasks of the unbalanced atrial targets for making a consistent optimization. It mainly forces the estimated joint distribution of LA and atrial scars to match the real ones. We evaluated the performance of our JAS-GAN on a 3D LGE CMR dataset with 192 scans. Compared with the state-of-the-art methods, our proposed approach yielded better segmentation performance (Average Dice Similarity Coefficient (DSC) values of 0.946 and 0.821 for LA and atrial scars, respectively), which indicated the effectiveness of our proposed approach for segmenting unbalanced atrial targets.
Simultaneous Left Atrium Anatomy and Scar Segmentations via Deep Learning in Multiview Information with Attention
Feb 02, 2020
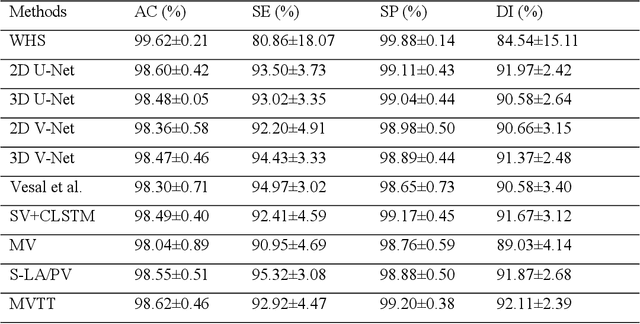

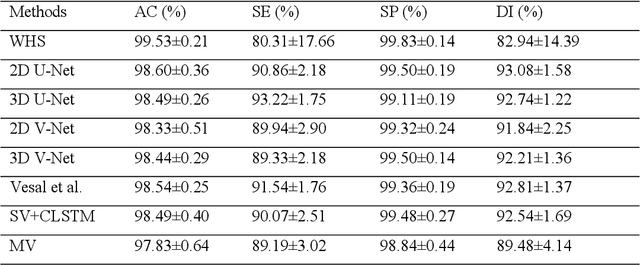
Abstract:Three-dimensional late gadolinium enhanced (LGE) cardiac MR (CMR) of left atrial scar in patients with atrial fibrillation (AF) has recently emerged as a promising technique to stratify patients, to guide ablation therapy and to predict treatment success. This requires a segmentation of the high intensity scar tissue and also a segmentation of the left atrium (LA) anatomy, the latter usually being derived from a separate bright-blood acquisition. Performing both segmentations automatically from a single 3D LGE CMR acquisition would eliminate the need for an additional acquisition and avoid subsequent registration issues. In this paper, we propose a joint segmentation method based on multiview two-task (MVTT) recursive attention model working directly on 3D LGE CMR images to segment the LA (and proximal pulmonary veins) and to delineate the scar on the same dataset. Using our MVTT recursive attention model, both the LA anatomy and scar can be segmented accurately (mean Dice score of 93% for the LA anatomy and 87% for the scar segmentations) and efficiently (~0.27 seconds to simultaneously segment the LA anatomy and scars directly from the 3D LGE CMR dataset with 60-68 2D slices). Compared to conventional unsupervised learning and other state-of-the-art deep learning based methods, the proposed MVTT model achieved excellent results, leading to an automatic generation of a patient-specific anatomical model combined with scar segmentation for patients in AF.
Discriminative Consistent Domain Generation for Semi-supervised Learning
Jul 24, 2019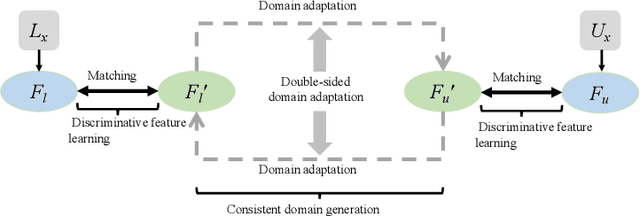
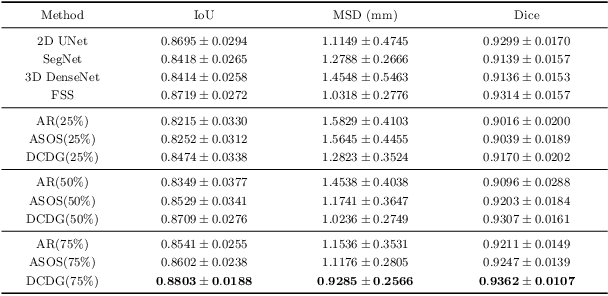
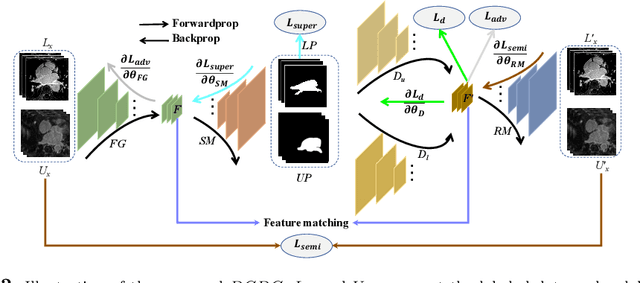
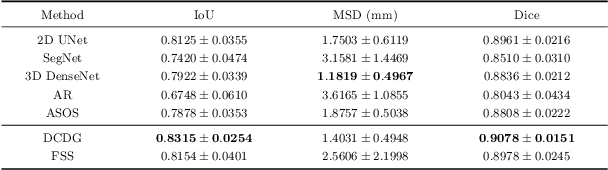
Abstract:Deep learning based task systems normally rely on a large amount of manually labeled training data, which is expensive to obtain and subject to operator variations. Moreover, it does not always hold that the manually labeled data and the unlabeled data are sitting in the same distribution. In this paper, we alleviate these problems by proposing a discriminative consistent domain generation (DCDG) approach to achieve a semi-supervised learning. The discriminative consistent domain is achieved by a double-sided domain adaptation. The double-sided domain adaptation aims to make a fusion of the feature spaces of labeled data and unlabeled data. In this way, we can fit the differences of various distributions between labeled data and unlabeled data. In order to keep the discriminativeness of generated consistent domain for the task learning, we apply an indirect learning for the double-sided domain adaptation. Based on the generated discriminative consistent domain, we can use the unlabeled data to learn the task model along with the labeled data via a consistent image generation. We demonstrate the performance of our proposed DCDG on the late gadolinium enhancement cardiac MRI (LGE-CMRI) images acquired from patients with atrial fibrillation in two clinical centers for the segmentation of the left atrium anatomy (LA) and proximal pulmonary veins (PVs). The experiments show that our semi-supervised approach achieves compelling segmentation results, which can prove the robustness of DCDG for the semi-supervised learning using the unlabeled data along with labeled data acquired from a single center or multicenter studies.
Atrial Scar Quantification via Multi-scale CNN in the Graph-cuts Framework
Feb 21, 2019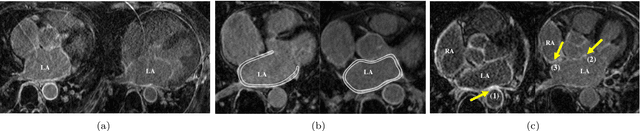
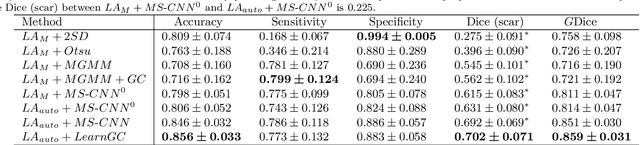
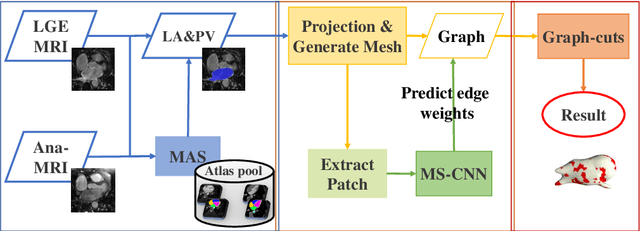

Abstract:Late gadolinium enhancement magnetic resonance imaging (LGE MRI) appears to be a promising alternative for scar assessment in patients with atrial fibrillation (AF). Automating the quantification and analysis of atrial scars can be challenging due to the low image quality. In this work, we propose a fully automated method based on the graph-cuts framework, where the potentials of the graph are learned on a surface mesh of the left atrium (LA) using a multi-scale convolutional neural network (MS-CNN). For validation, we have employed fifty-eight images with manual delineations. MS-CNN, which can efficiently incorporate both the local and global texture information of the images, has been shown to evidently improve the segmentation accuracy of the proposed graph-cuts based method. The segmentation could be further improved when the contribution between the t-link and n-link weights of the graph is balanced. The proposed method achieves a mean accuracy of 0.856 +- 0.033 and mean Dice score of 0.702 +- 0.071 for LA scar quantification. Compared with the conventional methods, which are based on the manual delineation of LA for initialization, our method is fully automatic and has demonstrated significantly better Dice score and accuracy (p < 0.01). The method is promising and can be useful in diagnosis and prognosis of AF.
Atrial scars segmentation via potential learning in the graph-cuts framework
Oct 22, 2018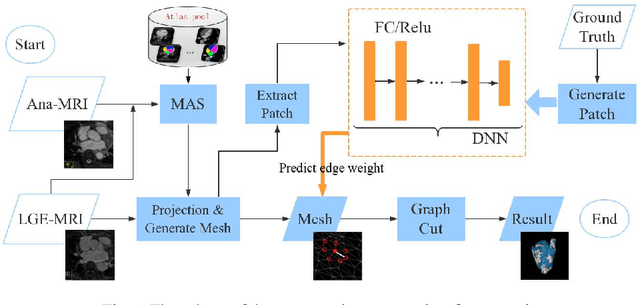
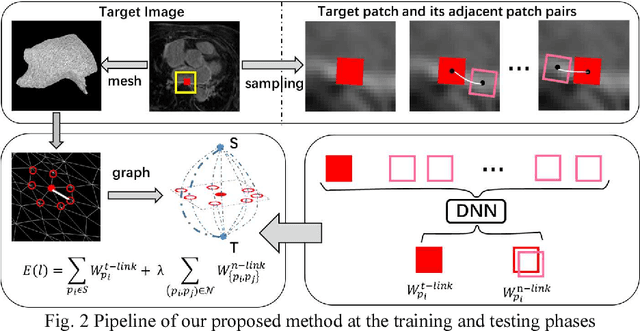
Abstract:Late Gadolinium Enhancement Magnetic Resonance Imaging (LGE MRI) emerged as a routine scan for patients with atrial fibrillation (AF). However, due to the low image quality automating the quantification and analysis of the atrial scars is challenging. In this study, we pro-posed a fully automated method based on the graph-cuts framework, where the potential of the graph is learned on a surface mesh of the left atrium (LA) using an equidistant projection and a Deep Neural Network (DNN). For validation, we employed 100 datasets with manual delineation. The results showed that the performance of the proposed method improved and converged with respect to the increased size of training patches, which provide important features of the structural and texture information learned by the DNN. The segmentation could be further improved when the contribution from the t-link and n-link is balanced, thanks to inter-relationship learned by the DNN for the graph-cuts algorithm. Compared with the published methods which mostly acquired manual delineation of the LA or LA wall, our method is fully automatic and demonstrated evidently better results with statistical significance. Finally, the accuracy of quantifying the scars assessed by the Dice score was 0.570. The results are promising and the method can be useful in diagnosis and prognosis of AF.
Atrial fibrosis quantification based on maximum likelihood estimator of multivariate images
Oct 22, 2018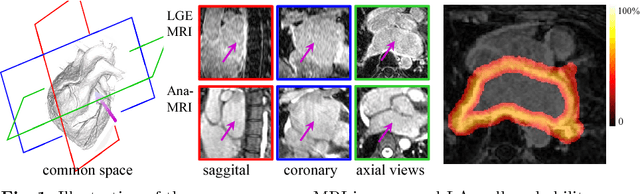

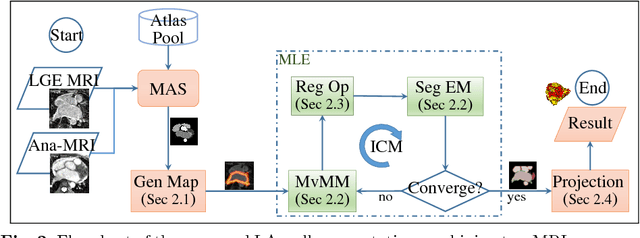
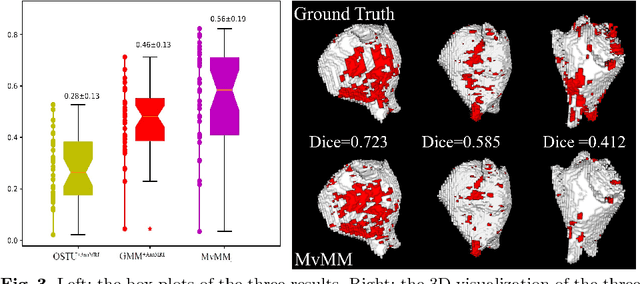
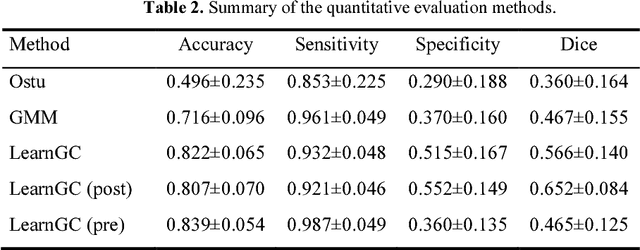
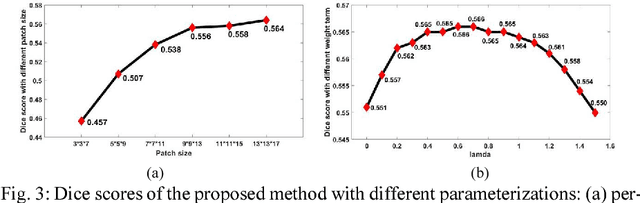
Abstract:We present a fully-automated segmentation and quantification of the left atrial (LA) fibrosis and scars combining two cardiac MRIs, one is the target late gadolinium-enhanced (LGE) image, and the other is an anatomical MRI from the same acquisition session. We formulate the joint distribution of images using a multivariate mixture model (MvMM), and employ the maximum likelihood estimator (MLE) for texture classification of the images simultaneously. The MvMM can also embed transformations assigned to the images to correct the misregistration. The iterated conditional mode algorithm is adopted for optimization. This method first extracts the anatomical shape of the LA, and then estimates a prior probability map. It projects the resulting segmentation onto the LA surface, for quantification and analysis of scarring. We applied the proposed method to 36 clinical data sets and obtained promising results (Accuracy: $0.809\pm .150$, Dice: $0.556\pm.187$). We compared the method with the conventional algorithms and showed an evidently and statistically better performance ($p<0.03$).
Adversarial and Perceptual Refinement for Compressed Sensing MRI Reconstruction
Jun 28, 2018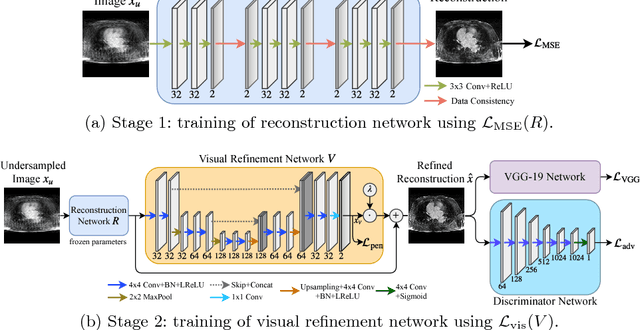
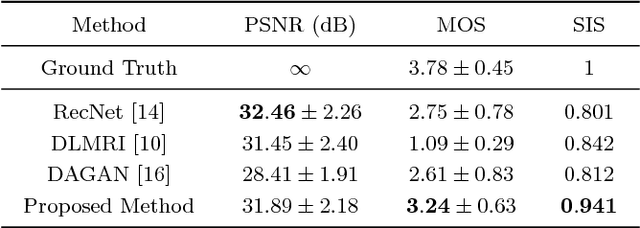
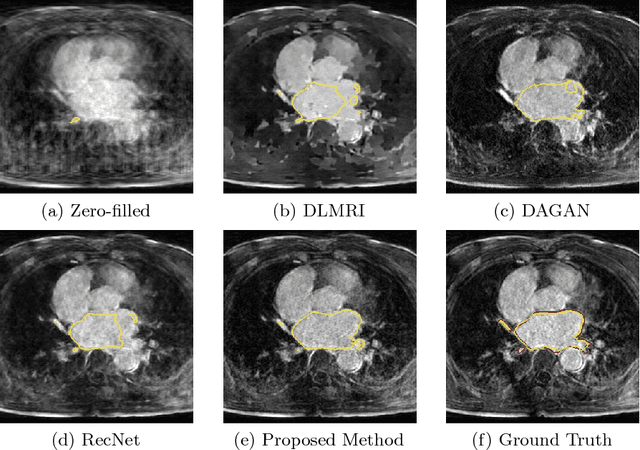
Abstract:Deep learning approaches have shown promising performance for compressed sensing-based Magnetic Resonance Imaging. While deep neural networks trained with mean squared error (MSE) loss functions can achieve high peak signal to noise ratio, the reconstructed images are often blurry and lack sharp details, especially for higher undersampling rates. Recently, adversarial and perceptual loss functions have been shown to achieve more visually appealing results. However, it remains an open question how to (1) optimally combine these loss functions with the MSE loss function and (2) evaluate such a perceptual enhancement. In this work, we propose a hybrid method, in which a visual refinement component is learnt on top of an MSE loss-based reconstruction network. In addition, we introduce a semantic interpretability score, measuring the visibility of the region of interest in both ground truth and reconstructed images, which allows us to objectively quantify the usefulness of the image quality for image post-processing and analysis. Applied on a large cardiac MRI dataset simulated with 8-fold undersampling, we demonstrate significant improvements ($p<0.01$) over the state-of-the-art in both a human observer study and the semantic interpretability score.
Multiview Two-Task Recursive Attention Model for Left Atrium and Atrial Scars Segmentation
Jun 12, 2018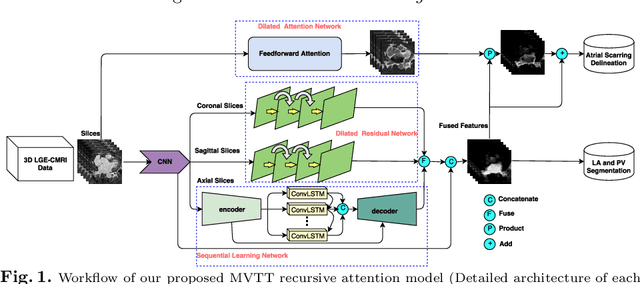
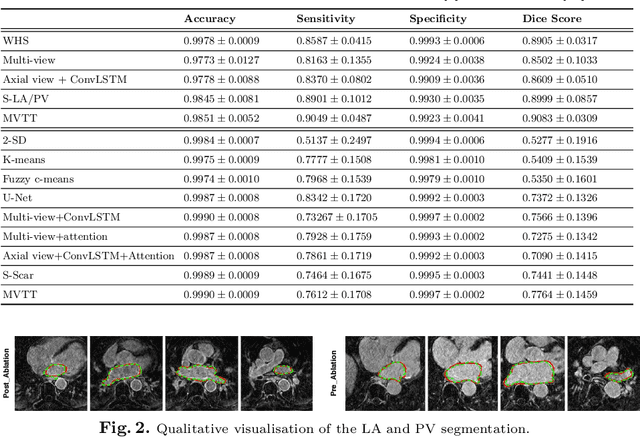
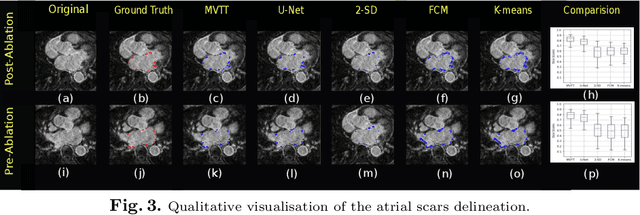
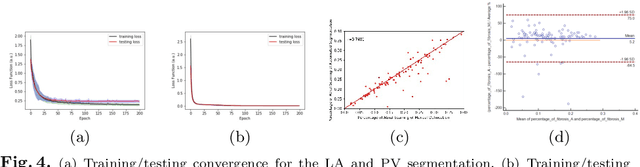
Abstract:Late Gadolinium Enhanced Cardiac MRI (LGE-CMRI) for detecting atrial scars in atrial fibrillation (AF) patients has recently emerged as a promising technique to stratify patients, guide ablation therapy and predict treatment success. Visualisation and quantification of scar tissues require a segmentation of both the left atrium (LA) and the high intensity scar regions from LGE-CMRI images. These two segmentation tasks are challenging due to the cancelling of healthy tissue signal, low signal-to-noise ratio and often limited image quality in these patients. Most approaches require manual supervision and/or a second bright-blood MRI acquisition for anatomical segmentation. Segmenting both the LA anatomy and the scar tissues automatically from a single LGE-CMRI acquisition is highly in demand. In this study, we proposed a novel fully automated multiview two-task (MVTT) recursive attention model working directly on LGE-CMRI images that combines a sequential learning and a dilated residual learning to segment the LA (including attached pulmonary veins) and delineate the atrial scars simultaneously via an innovative attention model. Compared to other state-of-the-art methods, the proposed MVTT achieves compelling improvement, enabling to generate a patient-specific anatomical and atrial scar assessment model.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge