Xiuquan Du
TC-Net: Triple Context Network for Automated Stroke Lesion Segmentation
Feb 28, 2022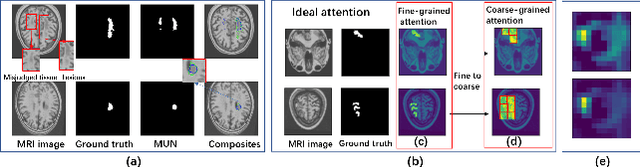
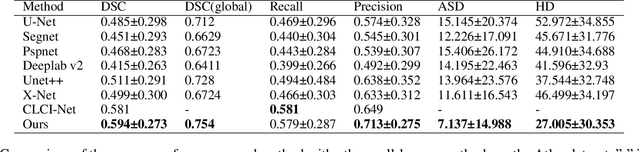
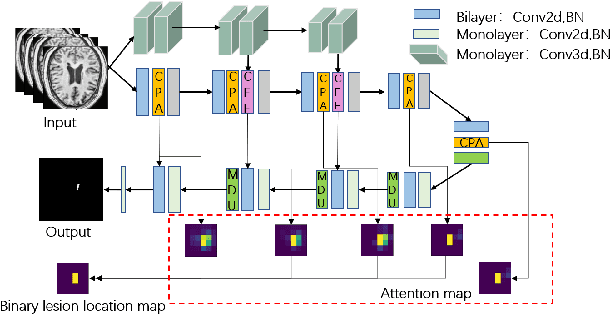
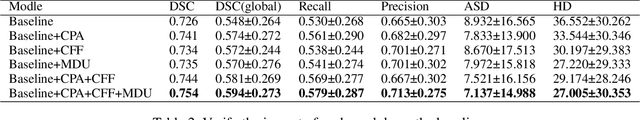
Abstract:Accurate lesion segmentation plays a key role in the clinical mapping of stroke. Convolutional neural network (CNN) approaches based on U-shaped structures have achieved remarkable performance in this task. However, the single-stage encoder-decoder unresolvable the inter-class similarity due to the inadequate utilization of contextual information, such as lesion-tissue similarity. In addition, most approaches use fine-grained spatial attention to capture spatial context information, yet fail to generate accurate attention maps in encoding stage and lack effective regularization. In this work, we propose a new network, Triple Context Network (TC-Net), with the capture of spatial contextual information as the core. We firstly design a coarse-grained patch attention module to generate patch-level attention maps in the encoding stage to distinguish targets from patches and learn target-specific detail features. Then, to enrich the representation of boundary information of these features, a cross-feature fusion module with global contextual information is explored to guide the selective aggregation of 2D and 3D feature maps, which compensates for the lack of boundary learning capability of 2D convolution. Finally, we use multi-scale deconvolution instead of linear interpolation to enhance the recovery of target space and boundary information in the decoding stage. Our network is evaluated on the open dataset ATLAS, achieving the highest DSC score of 0.594, Hausdorff distance of 27.005 mm, and average symmetry surface distance of 7.137 mm, where our proposed method outperforms other state-of-the-art methods.
Simultaneous Left Atrium Anatomy and Scar Segmentations via Deep Learning in Multiview Information with Attention
Feb 02, 2020
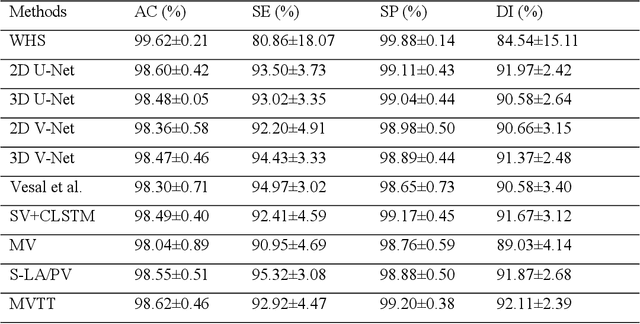

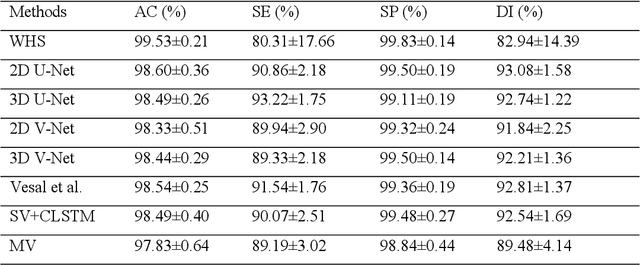
Abstract:Three-dimensional late gadolinium enhanced (LGE) cardiac MR (CMR) of left atrial scar in patients with atrial fibrillation (AF) has recently emerged as a promising technique to stratify patients, to guide ablation therapy and to predict treatment success. This requires a segmentation of the high intensity scar tissue and also a segmentation of the left atrium (LA) anatomy, the latter usually being derived from a separate bright-blood acquisition. Performing both segmentations automatically from a single 3D LGE CMR acquisition would eliminate the need for an additional acquisition and avoid subsequent registration issues. In this paper, we propose a joint segmentation method based on multiview two-task (MVTT) recursive attention model working directly on 3D LGE CMR images to segment the LA (and proximal pulmonary veins) and to delineate the scar on the same dataset. Using our MVTT recursive attention model, both the LA anatomy and scar can be segmented accurately (mean Dice score of 93% for the LA anatomy and 87% for the scar segmentations) and efficiently (~0.27 seconds to simultaneously segment the LA anatomy and scars directly from the 3D LGE CMR dataset with 60-68 2D slices). Compared to conventional unsupervised learning and other state-of-the-art deep learning based methods, the proposed MVTT model achieved excellent results, leading to an automatic generation of a patient-specific anatomical model combined with scar segmentation for patients in AF.
Multiview Two-Task Recursive Attention Model for Left Atrium and Atrial Scars Segmentation
Jun 12, 2018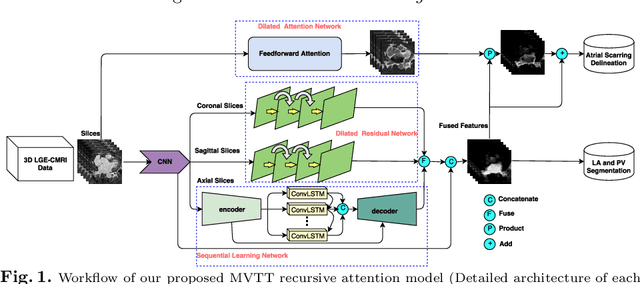
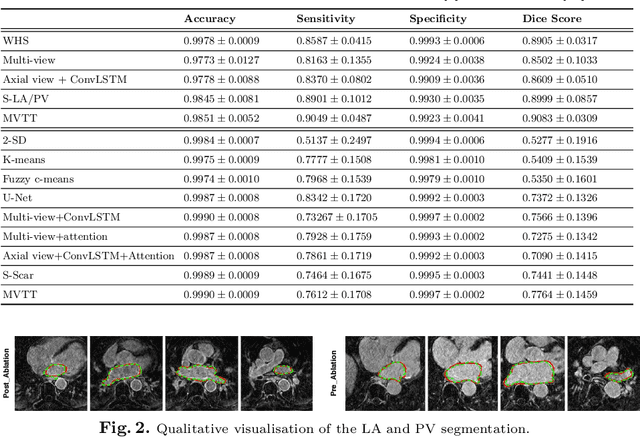
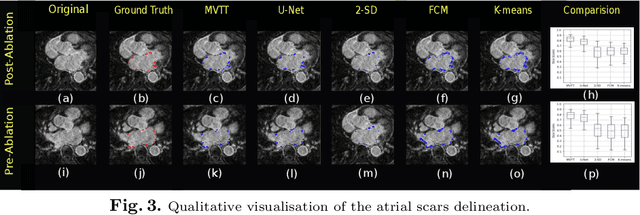
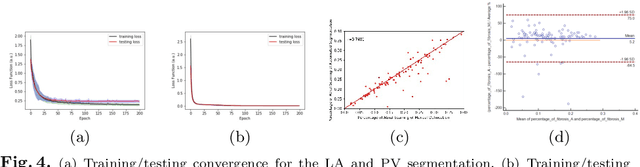
Abstract:Late Gadolinium Enhanced Cardiac MRI (LGE-CMRI) for detecting atrial scars in atrial fibrillation (AF) patients has recently emerged as a promising technique to stratify patients, guide ablation therapy and predict treatment success. Visualisation and quantification of scar tissues require a segmentation of both the left atrium (LA) and the high intensity scar regions from LGE-CMRI images. These two segmentation tasks are challenging due to the cancelling of healthy tissue signal, low signal-to-noise ratio and often limited image quality in these patients. Most approaches require manual supervision and/or a second bright-blood MRI acquisition for anatomical segmentation. Segmenting both the LA anatomy and the scar tissues automatically from a single LGE-CMRI acquisition is highly in demand. In this study, we proposed a novel fully automated multiview two-task (MVTT) recursive attention model working directly on LGE-CMRI images that combines a sequential learning and a dilated residual learning to segment the LA (including attached pulmonary veins) and delineate the atrial scars simultaneously via an innovative attention model. Compared to other state-of-the-art methods, the proposed MVTT achieves compelling improvement, enabling to generate a patient-specific anatomical and atrial scar assessment model.
Direct detection of pixel-level myocardial infarction areas via a deep-learning algorithm
Jun 10, 2017

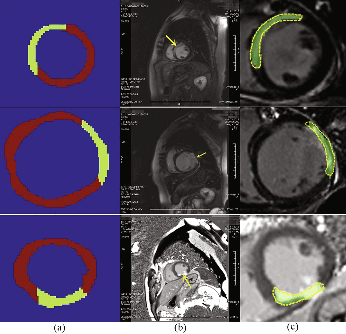
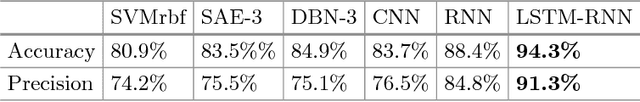
Abstract:Accurate detection of the myocardial infarction (MI) area is crucial for early diagnosis planning and follow-up management. In this study, we propose an end-to-end deep-learning algorithm framework (OF-RNN ) to accurately detect the MI area at the pixel level. Our OF-RNN consists of three different function layers: the heart localization layers, which can accurately and automatically crop the region-of-interest (ROI) sequences, including the left ventricle, using the whole cardiac magnetic resonance image sequences; the motion statistical layers, which are used to build a time-series architecture to capture two types of motion features (at the pixel-level) by integrating the local motion features generated by long short-term memory-recurrent neural networks and the global motion features generated by deep optical flows from the whole ROI sequence, which can effectively characterize myocardial physiologic function; and the fully connected discriminate layers, which use stacked auto-encoders to further learn these features, and they use a softmax classifier to build the correspondences from the motion features to the tissue identities (infarction or not) for each pixel. Through the seamless connection of each layer, our OF-RNN can obtain the area, position, and shape of the MI for each patient. Our proposed framework yielded an overall classification accuracy of 94.35% at the pixel level, from 114 clinical subjects. These results indicate the potential of our proposed method in aiding standardized MI assessments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge