Tim Leiner
Automatic Coronary Artery Plaque Quantification and CAD-RADS Prediction using Mesh Priors
Oct 17, 2023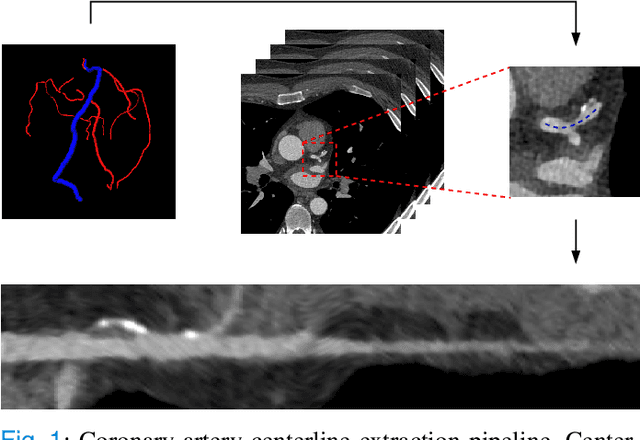
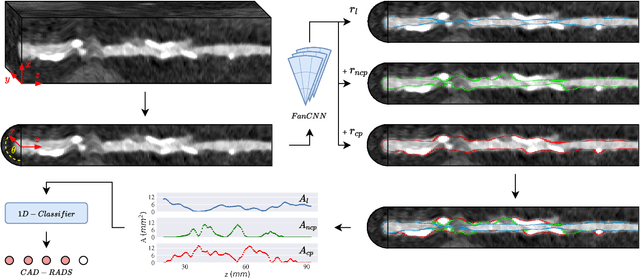
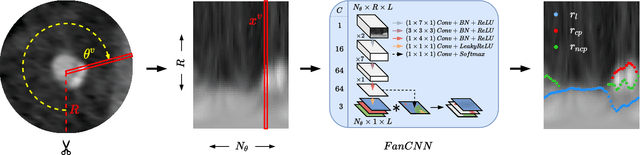
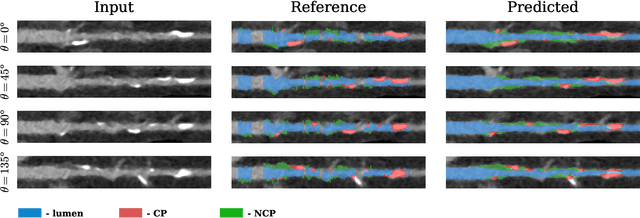
Abstract:Coronary artery disease (CAD) remains the leading cause of death worldwide. Patients with suspected CAD undergo coronary CT angiography (CCTA) to evaluate the risk of cardiovascular events and determine the treatment. Clinical analysis of coronary arteries in CCTA comprises the identification of atherosclerotic plaque, as well as the grading of any coronary artery stenosis typically obtained through the CAD-Reporting and Data System (CAD-RADS). This requires analysis of the coronary lumen and plaque. While voxel-wise segmentation is a commonly used approach in various segmentation tasks, it does not guarantee topologically plausible shapes. To address this, in this work, we propose to directly infer surface meshes for coronary artery lumen and plaque based on a centerline prior and use it in the downstream task of CAD-RADS scoring. The method is developed and evaluated using a total of 2407 CCTA scans. Our method achieved lesion-wise volume intraclass correlation coefficients of 0.98, 0.79, and 0.85 for calcified, non-calcified, and total plaque volume respectively. Patient-level CAD-RADS categorization was evaluated on a representative hold-out test set of 300 scans, for which the achieved linearly weighted kappa ($\kappa$) was 0.75. CAD-RADS categorization on the set of 658 scans from another hospital and scanner led to a $\kappa$ of 0.71. The results demonstrate that direct inference of coronary artery meshes for lumen and plaque is feasible, and allows for the automated prediction of routinely performed CAD-RADS categorization.
Decision making in cancer: Causal questions require causal answers
Sep 15, 2022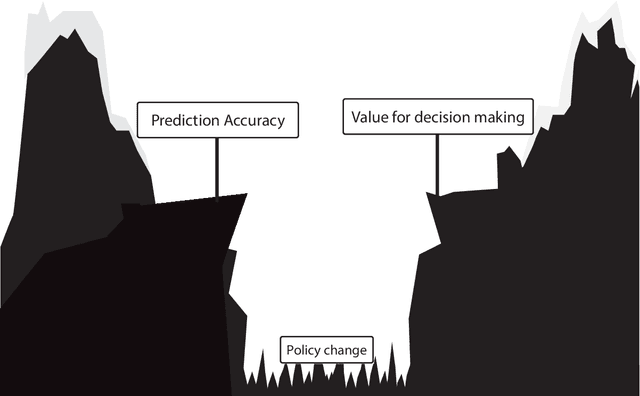
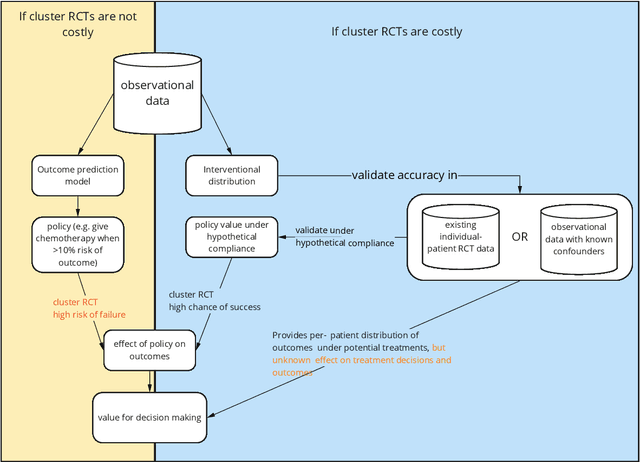
Abstract:Treatment decisions in cancer care are guided by treatment effect estimates from randomized controlled trials (RCTs). RCTs estimate the average effect of one treatment versus another in a certain population. However, treatments may not be equally effective for every patient in a population. Knowing the effectiveness of treatments tailored to specific patient and tumor characteristics would enable individualized treatment decisions. Getting tailored treatment effects by averaging outcomes in different patient subgroups in RCTs requires an unfeasible number of patients to have sufficient statistical power in all relevant subgroups for all possible treatments. The American Joint Committee on Cancer (AJCC) recommends that researchers develop outcome prediction models (OPMs) in an effort to individualize treatment decisions. OPMs sometimes called risk models or prognosis models, use patient and tumor characteristics to predict a patient outcome such as overall survival. The assumption is that the predictions are useful for treatment decisions using rules such as "prescribe chemotherapy only if the OPM predicts the patient has a high risk of recurrence". Recognizing the importance of reliable predictions, the AJCC published a checklist for OPMs to ensure dependable OPM prediction accuracy in the patient population for which the OPM was designed. However, accurate outcome predictions do not imply that these predictions yield good treatment decisions. In this perspective, we show that OPM rely on a fixed treatment policy which implies that OPM that were found to accurately predict outcomes in validation studies can still lead to patient harm when used to inform treatment decisions. We then give guidance on how to develop models that are useful for individualized treatment decisions and how to evaluate whether a model has value for decision-making.
AI-based Reconstruction for Fast MRI -- A Systematic Review and Meta-analysis
Dec 23, 2021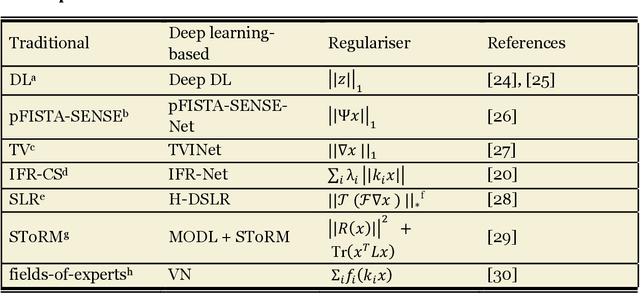
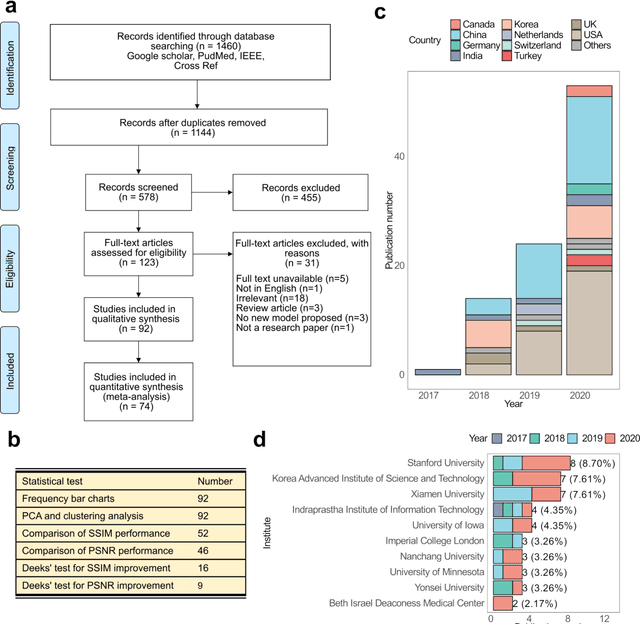
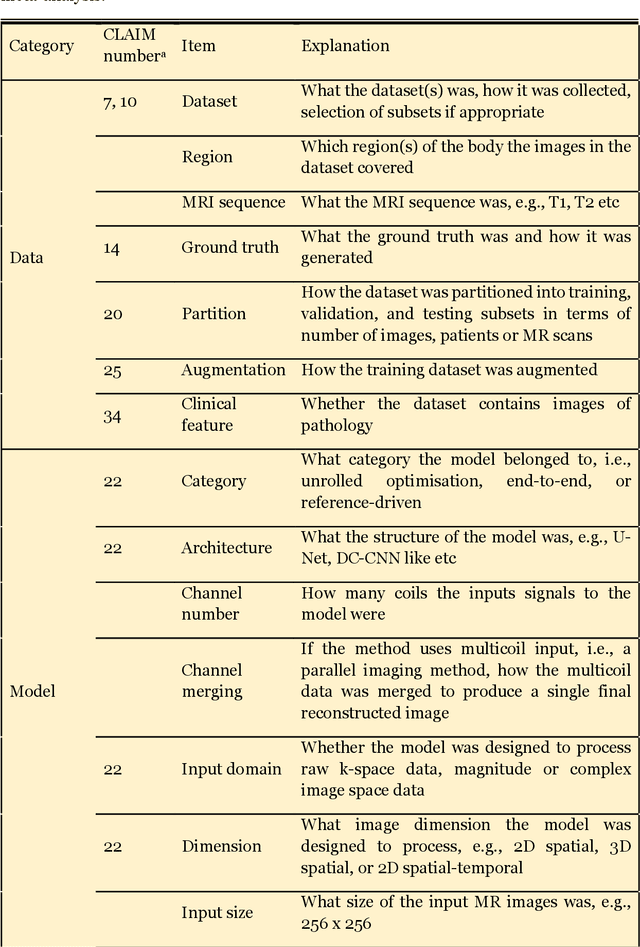
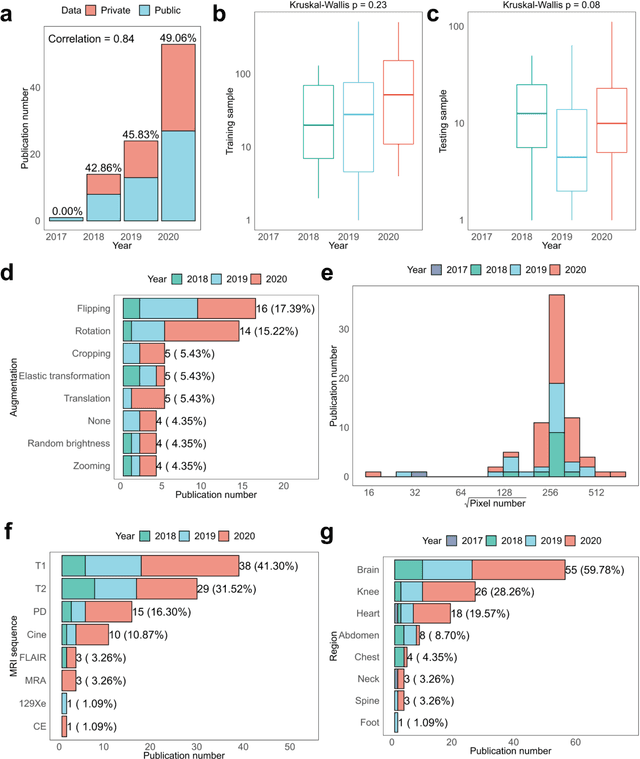
Abstract:Compressed sensing (CS) has been playing a key role in accelerating the magnetic resonance imaging (MRI) acquisition process. With the resurgence of artificial intelligence, deep neural networks and CS algorithms are being integrated to redefine the state of the art of fast MRI. The past several years have witnessed substantial growth in the complexity, diversity, and performance of deep learning-based CS techniques that are dedicated to fast MRI. In this meta-analysis, we systematically review the deep learning-based CS techniques for fast MRI, describe key model designs, highlight breakthroughs, and discuss promising directions. We have also introduced a comprehensive analysis framework and a classification system to assess the pivotal role of deep learning in CS-based acceleration for MRI.
Deep Learning from Dual-Energy Information for Whole-Heart Segmentation in Dual-Energy and Single-Energy Non-Contrast-Enhanced Cardiac CT
Aug 10, 2020



Abstract:Deep learning-based whole-heart segmentation in coronary CT angiography (CCTA) allows the extraction of quantitative imaging measures for cardiovascular risk prediction. Automatic extraction of these measures in patients undergoing only non-contrast-enhanced CT (NCCT) scanning would be valuable. In this work, we leverage information provided by a dual-layer detector CT scanner to obtain a reference standard in virtual non-contrast (VNC) CT images mimicking NCCT images, and train a 3D convolutional neural network (CNN) for the segmentation of VNC as well as NCCT images. Contrast-enhanced acquisitions on a dual-layer detector CT scanner were reconstructed into a CCTA and a perfectly aligned VNC image. In each CCTA image, manual reference segmentations of the left ventricular (LV) myocardium, LV cavity, right ventricle, left atrium, right atrium, ascending aorta, and pulmonary artery trunk were obtained and propagated to the corresponding VNC image. These VNC images and reference segmentations were used to train 3D CNNs for automatic segmentation in either VNC images or NCCT images. Automatic segmentations in VNC images showed good agreement with reference segmentations, with an average Dice similarity coefficient of 0.897 \pm 0.034 and an average symmetric surface distance of 1.42 \pm 0.45 mm. Volume differences [95% confidence interval] between automatic NCCT and reference CCTA segmentations were -19 [-67; 30] mL for LV myocardium, -25 [-78; 29] mL for LV cavity, -29 [-73; 14] mL for right ventricle, -20 [-62; 21] mL for left atrium, and -19 [-73; 34] mL for right atrium, respectively. In 214 (74%) NCCT images from an independent multi-vendor multi-center set, two observers agreed that the automatic segmentation was mostly accurate or better. This method might enable quantification of additional cardiac measures from NCCT images for improved cardiovascular risk prediction.
Deep Learning-Based Regression and Classification for Automatic Landmark Localization in Medical Images
Jul 10, 2020



Abstract:In this study, we propose a fast and accurate method to automatically localize anatomical landmarks in medical images. We employ a global-to-local localization approach using fully convolutional neural networks (FCNNs). First, a global FCNN localizes multiple landmarks through the analysis of image patches, performing regression and classification simultaneously. In regression, displacement vectors pointing from the center of image patches towards landmark locations are determined. In classification, presence of landmarks of interest in the patch is established. Global landmark locations are obtained by averaging the predicted displacement vectors, where the contribution of each displacement vector is weighted by the posterior classification probability of the patch that it is pointing from. Subsequently, for each landmark localized with global localization, local analysis is performed. Specialized FCNNs refine the global landmark locations by analyzing local sub-images in a similar manner, i.e. by performing regression and classification simultaneously and combining the results. Evaluation was performed through localization of 8 anatomical landmarks in CCTA scans, 2 landmarks in olfactory MR scans, and 19 landmarks in cephalometric X-rays. We demonstrate that the method performs similarly to a second observer and is able to localize landmarks in a diverse set of medical images, differing in image modality, image dimensionality, and anatomical coverage.
Combined analysis of coronary arteries and the left ventricular myocardium in cardiac CT angiography for detection of patients with functionally significant stenosis
Nov 10, 2019



Abstract:Treatment of patients with obstructive coronary artery disease is guided by the functional significance of a coronary artery stenosis. Fractional flow reserve (FFR), measured during invasive coronary angiography (ICA), is considered the gold standard to define the functional significance of a coronary stenosis. Here, we present a method for non-invasive detection of patients with functionally significant coronary artery stenosis, combining analysis of the coronary artery tree and the left ventricular (LV) myocardium in cardiac CT angiography (CCTA) images. We retrospectively collected CCTA scans of 126 patients who underwent invasive FFR measurements, to determine the functional significance of coronary stenoses. We combine our previous works for the analysis of the complete coronary artery tree and the LV myocardium: Coronary arteries are encoded by two disjoint convolutional autoencoders (CAEs) and the LV myocardium is characterized by a convolutional neural network (CNN) and a CAE. Thereafter, using the extracted encodings of all coronary arteries and the LV myocardium, patients are classified according to the presence of functionally significant stenosis, as defined by the invasively measured FFR. To handle the varying number of coronary arteries in a patient, the classification is formulated as a multiple instance learning problem and is performed using an attention-based neural network. Cross-validation experiments resulted in an average area under the receiver operating characteristic curve of $0.74 \pm 0.01$, and showed that the proposed combined analysis outperformed the analysis of the coronary arteries or the LV myocardium only. The results demonstrate the feasibility of combining the analyses of the complete coronary artery tree and the LV myocardium in CCTA images for the detection of patients with functionally significant stenosis in coronary arteries.
Graph Convolutional Networks for Coronary Artery Segmentation in Cardiac CT Angiography
Aug 14, 2019



Abstract:Detection of coronary artery stenosis in coronary CT angiography (CCTA) requires highly personalized surface meshes enclosing the coronary lumen. In this work, we propose to use graph convolutional networks (GCNs) to predict the spatial location of vertices in a tubular surface mesh that segments the coronary artery lumen. Predictions for individual vertex locations are based on local image features as well as on features of neighboring vertices in the mesh graph. The method was trained and evaluated using the publicly available Coronary Artery Stenoses Detection and Quantification Evaluation Framework. Surface meshes enclosing the full coronary artery tree were automatically extracted. A quantitative evaluation on 78 coronary artery segments showed that these meshes corresponded closely to reference annotations, with a Dice similarity coefficient of 0.75/0.73, a mean surface distance of 0.25/0.28 mm, and a Hausdorff distance of 1.53/1.86 mm in healthy/diseased vessel segments. The results showed that inclusion of mesh information in a GCN improves segmentation overlap and accuracy over a baseline model without interaction on the mesh. The results indicate that GCNs allow efficient extraction of coronary artery surface meshes and that the use of GCNs leads to regular and more accurate meshes.
Deep learning analysis of cardiac CT angiography for detection of coronary arteries with functionally significant stenosis
Jun 11, 2019



Abstract:In patients with obstructive coronary artery disease, the functional significance of a coronary artery stenosis needs to be determined to guide treatment. This is typically established through fractional flow reserve (FFR) measurement, performed during invasive coronary angiography (ICA). We present a method for automatic and non-invasive detection of functionally significant coronary artery stenosis, employing deep unsupervised analysis of complete coronary arteries in cardiac CT angiography (CCTA) images. We retrospectively collected CCTA scans of 187 patients, 137 of them underwent invasive FFR measurement in 192 different coronary arteries. These FFR measurements served as a reference standard for the functional significance of the coronary stenosis. The centerlines of the coronary arteries were extracted and used to reconstruct straightened multi-planar reformatted (MPR) volumes. To automatically identify arteries with functionally significant stenosis, each MPR volume was encoded into a fixed number of encodings using two disjoint 3D and 1D convolutional autoencoders performing spatial and sequential encodings, respectively. Thereafter, these encodings were employed to classify arteries according to the presence of functionally significant stenosis using a support vector machine classifier. The detection of functionally significant stenosis, evaluated using repeated cross-validation experiments, resulted in an area under the receiver operating characteristic curve of $0.81 \pm 0.02$ on the artery-level, and $0.87 \pm 0.02$ on the patient-level. The results demonstrate that automatic non-invasive detection of the functionally significant stenosis in coronary arteries, using characteristics of complete coronary arteries in CCTA images, is feasible. This could potentially reduce the number of patients that unnecessarily undergo ICA.
Direct Automatic Coronary Calcium Scoring in Cardiac and Chest CT
Feb 12, 2019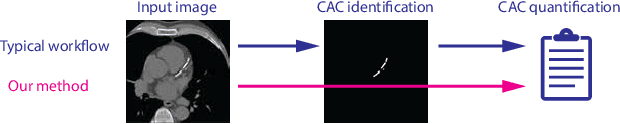
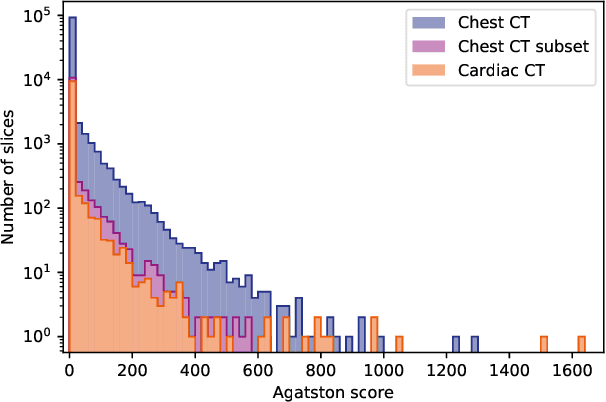
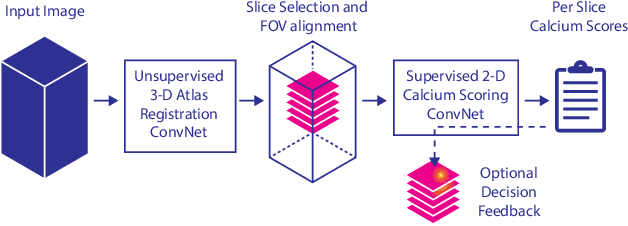
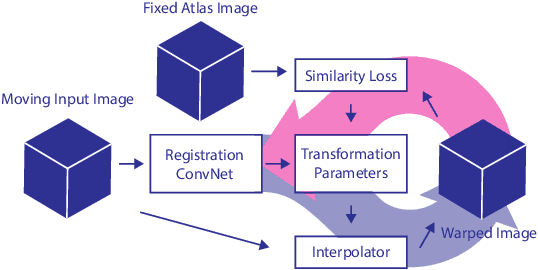
Abstract:Cardiovascular disease (CVD) is the global leading cause of death. A strong risk factor for CVD events is the amount of coronary artery calcium (CAC). To meet demands of the increasing interest in quantification of CAC, i.e. coronary calcium scoring, especially as an unrequested finding for screening and research, automatic methods have been proposed. Current automatic calcium scoring methods are relatively computationally expensive and only provide scores for one type of CT. To address this, we propose a computationally efficient method that employs two ConvNets: the first performs registration to align the fields of view of input CTs and the second performs direct regression of the calcium score, thereby circumventing time-consuming intermediate CAC segmentation. Optional decision feedback provides insight in the regions that contributed to the calcium score. Experiments were performed using 903 cardiac CT and 1,687 chest CT scans. The method predicted calcium scores in less than 0.3 s. Intra-class correlation coefficient between predicted and manual calcium scores was 0.98 for both cardiac and chest CT. The method showed almost perfect agreement between automatic and manual CVD risk categorization in both datasets, with a linearly weighted Cohen's kappa of 0.95 in cardiac CT and 0.93 in chest CT. Performance is similar to that of state-of-the-art methods, but the proposed method is hundreds of times faster. By providing visual feedback, insight is given in the decision process, making it readily implementable in clinical and research settings.
Coronary Artery Centerline Extraction in Cardiac CT Angiography Using a CNN-Based Orientation Classifier
Oct 24, 2018



Abstract:Coronary artery centerline extraction in cardiac CT angiography (CCTA) images is a prerequisite for evaluation of stenoses and atherosclerotic plaque. We propose an algorithm that extracts coronary artery centerlines in CCTA using a convolutional neural network (CNN). A 3D dilated CNN is trained to predict the most likely direction and radius of an artery at any given point in a CCTA image based on a local image patch. Starting from a single seed point placed manually or automatically anywhere in a coronary artery, a tracker follows the vessel centerline in two directions using the predictions of the CNN. Tracking is terminated when no direction can be identified with high certainty. The CNN was trained using 32 manually annotated centerlines in a training set consisting of 8 CCTA images provided in the MICCAI 2008 Coronary Artery Tracking Challenge (CAT08). Evaluation using 24 test images of the CAT08 challenge showed that extracted centerlines had an average overlap of 93.7% with 96 manually annotated reference centerlines. Extracted centerline points were highly accurate, with an average distance of 0.21 mm to reference centerline points. In a second test set consisting of 50 CCTA scans, 5,448 markers in the coronary arteries were used as seed points to extract single centerlines. This showed strong correspondence between extracted centerlines and manually placed markers. In a third test set containing 36 CCTA scans, fully automatic seeding and centerline extraction led to extraction of on average 92% of clinically relevant coronary artery segments. The proposed method is able to accurately and efficiently determine the direction and radius of coronary arteries. The method can be trained with limited training data, and once trained allows fast automatic or interactive extraction of coronary artery trees from CCTA images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge