Ivana Isgum
Evaluation of Mean Shift, ComBat, and CycleGAN for Harmonizing Brain Connectivity Matrices Across Sites
Jan 24, 2024



Abstract:Connectivity matrices derived from diffusion MRI (dMRI) provide an interpretable and generalizable way of understanding the human brain connectome. However, dMRI suffers from inter-site and between-scanner variation, which impedes analysis across datasets to improve robustness and reproducibility of results. To evaluate different harmonization approaches on connectivity matrices, we compared graph measures derived from these matrices before and after applying three harmonization techniques: mean shift, ComBat, and CycleGAN. The sample comprises 168 age-matched, sex-matched normal subjects from two studies: the Vanderbilt Memory and Aging Project (VMAP) and the Biomarkers of Cognitive Decline Among Normal Individuals (BIOCARD). First, we plotted the graph measures and used coefficient of variation (CoV) and the Mann-Whitney U test to evaluate different methods' effectiveness in removing site effects on the matrices and the derived graph measures. ComBat effectively eliminated site effects for global efficiency and modularity and outperformed the other two methods. However, all methods exhibited poor performance when harmonizing average betweenness centrality. Second, we tested whether our harmonization methods preserved correlations between age and graph measures. All methods except for CycleGAN in one direction improved correlations between age and global efficiency and between age and modularity from insignificant to significant with p-values less than 0.05.
Inter-vendor harmonization of Computed Tomography (CT) reconstruction kernels using unpaired image translation
Sep 22, 2023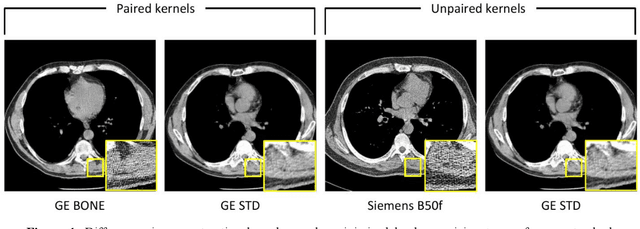
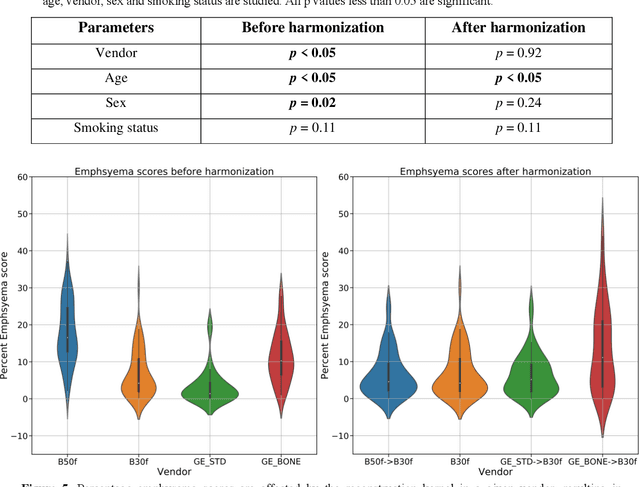
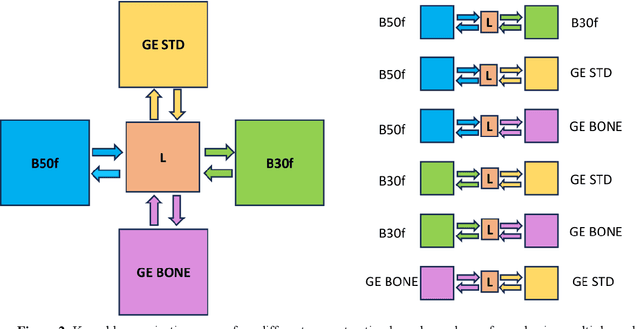
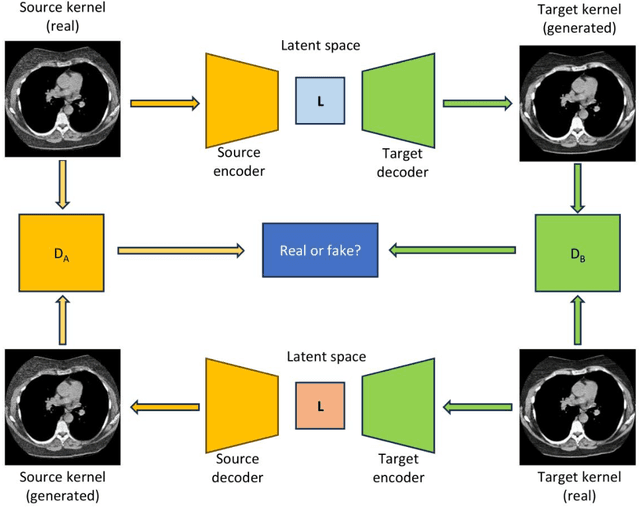
Abstract:The reconstruction kernel in computed tomography (CT) generation determines the texture of the image. Consistency in reconstruction kernels is important as the underlying CT texture can impact measurements during quantitative image analysis. Harmonization (i.e., kernel conversion) minimizes differences in measurements due to inconsistent reconstruction kernels. Existing methods investigate harmonization of CT scans in single or multiple manufacturers. However, these methods require paired scans of hard and soft reconstruction kernels that are spatially and anatomically aligned. Additionally, a large number of models need to be trained across different kernel pairs within manufacturers. In this study, we adopt an unpaired image translation approach to investigate harmonization between and across reconstruction kernels from different manufacturers by constructing a multipath cycle generative adversarial network (GAN). We use hard and soft reconstruction kernels from the Siemens and GE vendors from the National Lung Screening Trial dataset. We use 50 scans from each reconstruction kernel and train a multipath cycle GAN. To evaluate the effect of harmonization on the reconstruction kernels, we harmonize 50 scans each from Siemens hard kernel, GE soft kernel and GE hard kernel to a reference Siemens soft kernel (B30f) and evaluate percent emphysema. We fit a linear model by considering the age, smoking status, sex and vendor and perform an analysis of variance (ANOVA) on the emphysema scores. Our approach minimizes differences in emphysema measurement and highlights the impact of age, sex, smoking status and vendor on emphysema quantification.
3D Convolutional Neural Networks Image Registration Based on Efficient Supervised Learning from Artificial Deformations
Aug 27, 2019



Abstract:We propose a supervised nonrigid image registration method, trained using artificial displacement vector fields (DVF), for which we propose and compare three network architectures. The artificial DVFs allow training in a fully supervised and voxel-wise dense manner, but without the cost usually associated with the creation of densely labeled data. We propose a scheme to artificially generate DVFs, and for chest CT registration augment these with simulated respiratory motion. The proposed architectures are embedded in a multi-stage approach, to increase the capture range of the proposed networks in order to more accurately predict larger displacements. The proposed method, RegNet, is evaluated on multiple databases of chest CT scans and achieved a target registration error of 2.32 $\pm$ 5.33 mm and 1.86 $\pm$ 2.12 mm on SPREAD and DIR-Lab-4DCT studies, respectively. The average inference time of RegNet with two stages is about 2.2 s.
Direct Automatic Coronary Calcium Scoring in Cardiac and Chest CT
Feb 12, 2019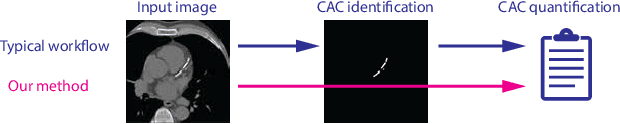
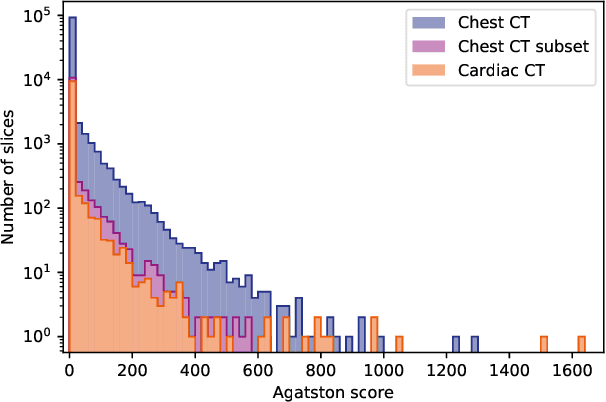
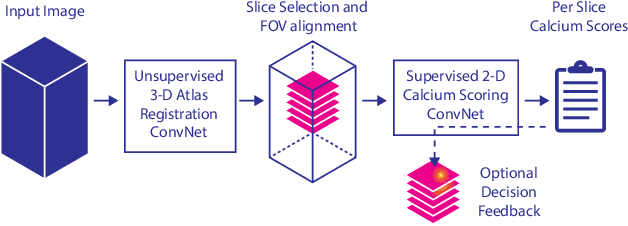
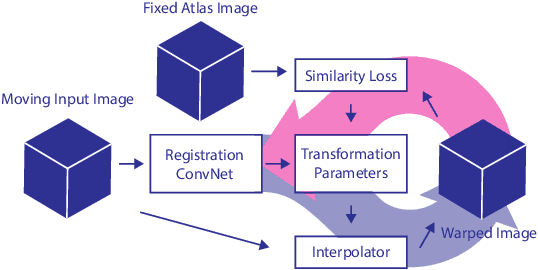
Abstract:Cardiovascular disease (CVD) is the global leading cause of death. A strong risk factor for CVD events is the amount of coronary artery calcium (CAC). To meet demands of the increasing interest in quantification of CAC, i.e. coronary calcium scoring, especially as an unrequested finding for screening and research, automatic methods have been proposed. Current automatic calcium scoring methods are relatively computationally expensive and only provide scores for one type of CT. To address this, we propose a computationally efficient method that employs two ConvNets: the first performs registration to align the fields of view of input CTs and the second performs direct regression of the calcium score, thereby circumventing time-consuming intermediate CAC segmentation. Optional decision feedback provides insight in the regions that contributed to the calcium score. Experiments were performed using 903 cardiac CT and 1,687 chest CT scans. The method predicted calcium scores in less than 0.3 s. Intra-class correlation coefficient between predicted and manual calcium scores was 0.98 for both cardiac and chest CT. The method showed almost perfect agreement between automatic and manual CVD risk categorization in both datasets, with a linearly weighted Cohen's kappa of 0.95 in cardiac CT and 0.93 in chest CT. Performance is similar to that of state-of-the-art methods, but the proposed method is hundreds of times faster. By providing visual feedback, insight is given in the decision process, making it readily implementable in clinical and research settings.
Response monitoring of breast cancer on DCE-MRI using convolutional neural network-generated seed points and constrained volume growing
Nov 22, 2018Abstract:Response of breast cancer to neoadjuvant chemotherapy (NAC) can be monitored using the change in visible tumor on magnetic resonance imaging (MRI). In our current workflow, seed points are manually placed in areas of enhancement likely to contain cancer. A constrained volume growing method uses these manually placed seed points as input and generates a tumor segmentation. This method is rigorously validated using complete pathological embedding. In this study, we propose to exploit deep learning for fast and automatic seed point detection, replacing manual seed point placement in our existing and well-validated workflow. The seed point generator was developed in early breast cancer patients with pathology-proven segmentations (N=100), operated shortly after MRI. It consisted of an ensemble of three independently trained fully convolutional dilated neural networks that classified breast voxels as tumor or non-tumor. Subsequently, local maxima were used as seed points for volume growing in patients receiving NAC (N=10). The percentage of tumor volume change was evaluated against semi-automatic segmentations. The primary cancer was localized in 95% of the tumors at the cost of 0.9 false positive per patient. False positives included focally enhancing regions of unknown origin and parts of the intramammary blood vessels. Volume growing from the seed points showed a median tumor volume decrease of 70% (interquartile range: 50%-77%), comparable to the semi-automatic segmentations (median: 70%, interquartile range 23%-76%). To conclude, a fast and automatic seed point generator was developed, fully automating a well-validated semi-automatic workflow for response monitoring of breast cancer to neoadjuvant chemotherapy.
Automatic Segmentation of Thoracic Aorta Segments in Low-Dose Chest CT
Oct 09, 2018Abstract:Morphological analysis and identification of pathologies in the aorta are important for cardiovascular diagnosis and risk assessment in patients. Manual annotation is time-consuming and cumbersome in CT scans acquired without contrast enhancement and with low radiation dose. Hence, we propose an automatic method to segment the ascending aorta, the aortic arch and the thoracic descending aorta in low-dose chest CT without contrast enhancement. Segmentation was performed using a dilated convolutional neural network (CNN), with a receptive field of 131X131 voxels, that classified voxels in axial, coronal and sagittal image slices. To obtain a final segmentation, the obtained probabilities of the three planes were averaged per class, and voxels were subsequently assigned to the class with the highest class probability. Two-fold cross-validation experiments were performed where ten scans were used to train the network and another ten to evaluate the performance. Dice coefficients of 0.83, 0.86 and 0.88, and Average Symmetrical Surface Distances (ASSDs) of 2.44, 1.56 and 1.87 mm were obtained for the ascending aorta, the aortic arch, and the descending aorta, respectively. The results indicate that the proposed method could be used in large-scale studies analyzing the anatomical location of pathology and morphology of the thoracic aorta.
A Deep Learning Framework for Unsupervised Affine and Deformable Image Registration
Sep 17, 2018



Abstract:Image registration, the process of aligning two or more images, is the core technique of many (semi-)automatic medical image analysis tasks. Recent studies have shown that deep learning methods, notably convolutional neural networks (ConvNets), can be used for image registration. Thus far training of ConvNets for registration was supervised using predefined example registrations. However, obtaining example registrations is not trivial. To circumvent the need for predefined examples, and thereby to increase convenience of training ConvNets for image registration, we propose the Deep Learning Image Registration (DLIR) framework for \textit{unsupervised} affine and deformable image registration. In the DLIR framework ConvNets are trained for image registration by exploiting image similarity analogous to conventional intensity-based image registration. After a ConvNet has been trained with the DLIR framework, it can be used to register pairs of unseen images in one shot. We propose flexible ConvNets designs for affine image registration and for deformable image registration. By stacking multiple of these ConvNets into a larger architecture, we are able to perform coarse-to-fine image registration. We show for registration of cardiac cine MRI and registration of chest CT that performance of the DLIR framework is comparable to conventional image registration while being several orders of magnitude faster.
A Recurrent CNN for Automatic Detection and Classification of Coronary Artery Plaque and Stenosis in Coronary CT Angiography
Aug 20, 2018



Abstract:Various types of atherosclerotic plaque and varying grades of stenosis could lead to different management of patients with coronary artery disease. Therefore, it is crucial to detect and classify the type of coronary artery plaque, as well as to detect and determine the degree of coronary artery stenosis. This study includes retrospectively collected clinically obtained coronary CT angiography (CCTA) scans of 163 patients. To perform automatic analysis for coronary artery plaque and stenosis classification, a multi-task recurrent convolutional neural network is applied on multi-planar reformatted (MPR) images of the coronary arteries. First, a 3D convolutional neural network is utilized to extract features along the coronary artery. Subsequently, the extracted features are aggregated by a recurrent neural network that performs two simultaneous multi-class classification tasks. In the first task, the network detects and characterizes the type of the coronary artery plaque (no plaque, non-calcified, mixed, calcified). In the second task, the network detects and determines the anatomical significance of the coronary artery stenosis (no stenosis, non-significant i.e. <50% luminal narrowing, significant i.e. >50% luminal narrowing). For detection and classification of coronary plaque, the method achieved an accuracy of 0.77. For detection and classification of stenosis, the method achieved an accuracy of 0.80. The results demonstrate that automatic detection and classification of coronary artery plaque and stenosis are feasible. This may enable automated triage of patients to those without coronary plaque and those with coronary plaque and stenosis in need for further cardiovascular workup.
Blood Vessel Geometry Synthesis using Generative Adversarial Networks
Apr 12, 2018



Abstract:Computationally synthesized blood vessels can be used for training and evaluation of medical image analysis applications. We propose a deep generative model to synthesize blood vessel geometries, with an application to coronary arteries in cardiac CT angiography (CCTA). In the proposed method, a Wasserstein generative adversarial network (GAN) consisting of a generator and a discriminator network is trained. While the generator tries to synthesize realistic blood vessel geometries, the discriminator tries to distinguish synthesized geometries from those of real blood vessels. Both real and synthesized blood vessel geometries are parametrized as 1D signals based on the central vessel axis. The generator can optionally be provided with an attribute vector to synthesize vessels with particular characteristics. The GAN was optimized using a reference database with parametrizations of 4,412 real coronary artery geometries extracted from CCTA scans. After training, plausible coronary artery geometries could be synthesized based on random vectors sampled from a latent space. A qualitative analysis showed strong similarities between real and synthesized coronary arteries. A detailed analysis of the latent space showed that the diversity present in coronary artery anatomy was accurately captured by the generator. Results show that Wasserstein generative adversarial networks can be used to synthesize blood vessel geometries.
Direct and Real-Time Cardiovascular Risk Prediction
Dec 08, 2017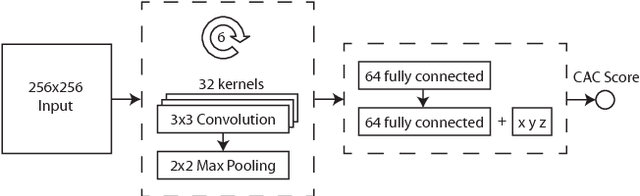
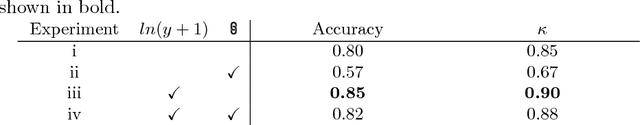
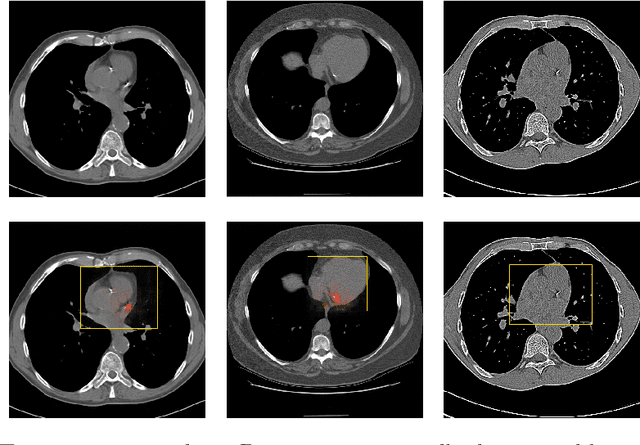
Abstract:Coronary artery calcium (CAC) burden quantified in low-dose chest CT is a predictor of cardiovascular events. We propose an automatic method for CAC quantification, circumventing intermediate segmentation of CAC. The method determines a bounding box around the heart using a ConvNet for localization. Subsequently, a dedicated ConvNet analyzes axial slices within the bounding boxes to determine CAC quantity by regression. A dataset of 1,546 baseline CT scans was used from the National Lung Screening Trial with manually identified CAC. The method achieved an ICC of 0.98 between manual reference and automatically obtained Agatston scores. Stratification of subjects into five cardiovascular risk categories resulted in an accuracy of 85\% and Cohen's linearly weighted $\kappa$ of 0.90. The results demonstrate that real-time quantification of CAC burden in chest CT without the need for segmentation of CAC is possible.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge