Nils Hampe
Automatic Coronary Artery Plaque Quantification and CAD-RADS Prediction using Mesh Priors
Oct 17, 2023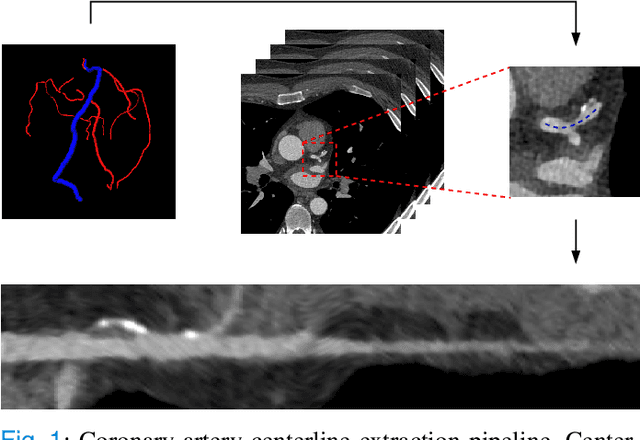
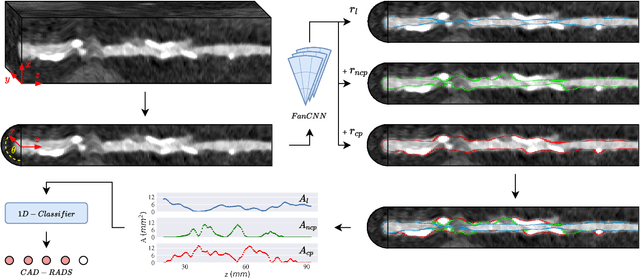
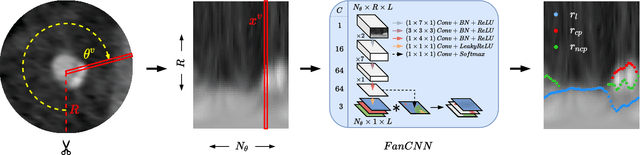
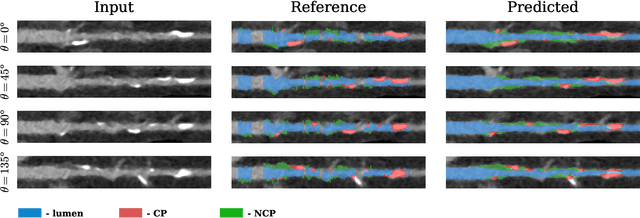
Abstract:Coronary artery disease (CAD) remains the leading cause of death worldwide. Patients with suspected CAD undergo coronary CT angiography (CCTA) to evaluate the risk of cardiovascular events and determine the treatment. Clinical analysis of coronary arteries in CCTA comprises the identification of atherosclerotic plaque, as well as the grading of any coronary artery stenosis typically obtained through the CAD-Reporting and Data System (CAD-RADS). This requires analysis of the coronary lumen and plaque. While voxel-wise segmentation is a commonly used approach in various segmentation tasks, it does not guarantee topologically plausible shapes. To address this, in this work, we propose to directly infer surface meshes for coronary artery lumen and plaque based on a centerline prior and use it in the downstream task of CAD-RADS scoring. The method is developed and evaluated using a total of 2407 CCTA scans. Our method achieved lesion-wise volume intraclass correlation coefficients of 0.98, 0.79, and 0.85 for calcified, non-calcified, and total plaque volume respectively. Patient-level CAD-RADS categorization was evaluated on a representative hold-out test set of 300 scans, for which the achieved linearly weighted kappa ($\kappa$) was 0.75. CAD-RADS categorization on the set of 658 scans from another hospital and scanner led to a $\kappa$ of 0.71. The results demonstrate that direct inference of coronary artery meshes for lumen and plaque is feasible, and allows for the automated prediction of routinely performed CAD-RADS categorization.
Deep Learning-Based Prediction of Fractional Flow Reserve along the Coronary Artery
Aug 09, 2023Abstract:Functionally significant coronary artery disease (CAD) is caused by plaque buildup in the coronary arteries, potentially leading to narrowing of the arterial lumen, i.e. coronary stenosis, that significantly obstructs blood flow to the myocardium. The current reference for establishing the presence of a functionally significant stenosis is invasive fractional flow reserve (FFR) measurement. To avoid invasive measurements, non-invasive prediction of FFR from coronary CT angiography (CCTA) has emerged. For this, machine learning approaches, characterized by fast inference, are increasingly developed. However, these methods predict a single FFR value per artery i.e. they don't provide information about the stenosis location or treatment strategy. We propose a deep learning-based method to predict the FFR along the artery from CCTA scans. This study includes CCTA images of 110 patients who underwent invasive FFR pullback measurement in 112 arteries. First, a multi planar reconstruction (MPR) of the artery is fed to a variational autoencoder to characterize the artery, i.e. through the lumen area and unsupervised artery encodings. Thereafter, a convolutional neural network (CNN) predicts the FFR along the artery. The CNN is supervised by multiple loss functions, notably a loss function inspired by the Earth Mover's Distance (EMD) to predict the correct location of FFR drops and a histogram-based loss to explicitly supervise the slope of the FFR curve. To train and evaluate our model, eight-fold cross-validation was performed. The resulting FFR curves show good agreement with the reference allowing the distinction between diffuse and focal CAD distributions in most cases. Quantitative evaluation yielded a mean absolute difference in the area under the FFR pullback curve (AUPC) of 1.7. The method may pave the way towards fast, accurate, automatic prediction of FFR along the artery from CCTA.
AI for Calcium Scoring
May 24, 2021



Abstract:Calcium scoring, a process in which arterial calcifications are detected and quantified in CT, is valuable in estimating the risk of cardiovascular disease events. Especially when used to quantify the extent of calcification in the coronary arteries, it is a strong and independent predictor of coronary heart disease events. Advances in artificial intelligence (AI)-based image analysis have produced a multitude of automatic calcium scoring methods. While most early methods closely follow standard calcium scoring accepted in clinic, recent approaches extend this procedure to enable faster or more reproducible calcium scoring. This chapter provides an introduction to AI for calcium scoring, and an overview of the developed methods and their applications. We conclude with a discussion on AI methods in calcium scoring and propose potential directions for future research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge