Carlos Collet
A Diffusion Model for Simulation Ready Coronary Anatomy with Morpho-skeletal Control
Jul 23, 2024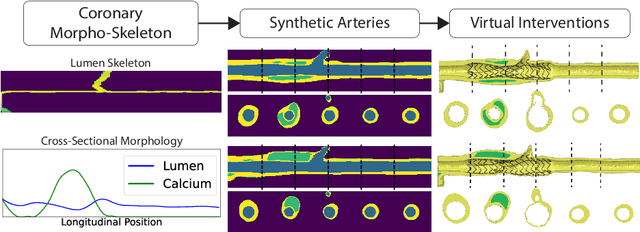
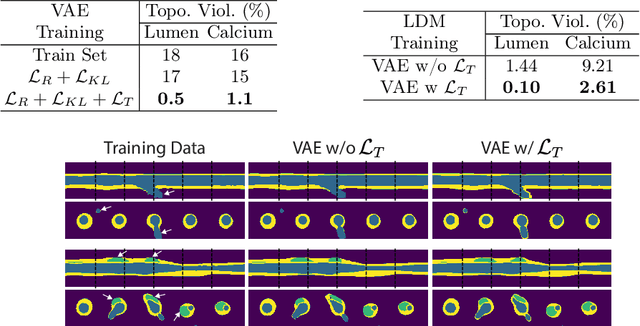
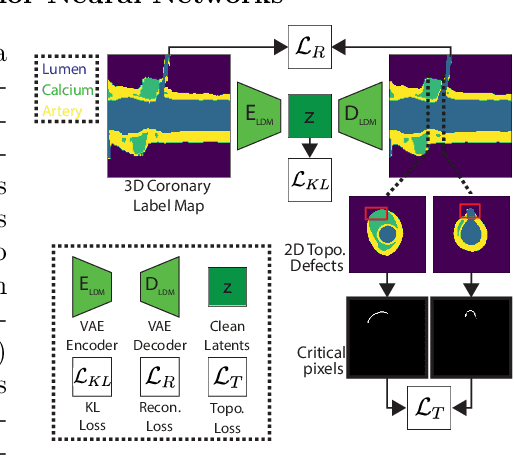
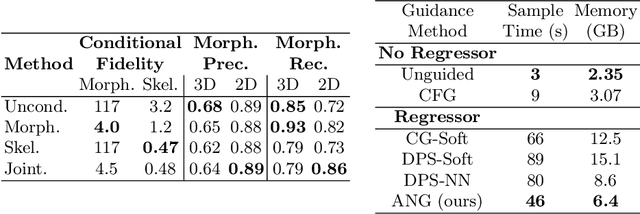
Abstract:Virtual interventions enable the physics-based simulation of device deployment within coronary arteries. This framework allows for counterfactual reasoning by deploying the same device in different arterial anatomies. However, current methods to create such counterfactual arteries face a trade-off between controllability and realism. In this study, we investigate how Latent Diffusion Models (LDMs) can custom synthesize coronary anatomy for virtual intervention studies based on mid-level anatomic constraints such as topological validity, local morphological shape, and global skeletal structure. We also extend diffusion model guidance strategies to the context of morpho-skeletal conditioning and propose a novel guidance method for continuous attributes that adaptively updates the negative guiding condition throughout sampling. Our framework enables the generation and editing of coronary anatomy in a controllable manner, allowing device designers to derive mechanistic insights regarding anatomic variation and simulated device deployment.
Deep Learning-Based Prediction of Fractional Flow Reserve along the Coronary Artery
Aug 09, 2023Abstract:Functionally significant coronary artery disease (CAD) is caused by plaque buildup in the coronary arteries, potentially leading to narrowing of the arterial lumen, i.e. coronary stenosis, that significantly obstructs blood flow to the myocardium. The current reference for establishing the presence of a functionally significant stenosis is invasive fractional flow reserve (FFR) measurement. To avoid invasive measurements, non-invasive prediction of FFR from coronary CT angiography (CCTA) has emerged. For this, machine learning approaches, characterized by fast inference, are increasingly developed. However, these methods predict a single FFR value per artery i.e. they don't provide information about the stenosis location or treatment strategy. We propose a deep learning-based method to predict the FFR along the artery from CCTA scans. This study includes CCTA images of 110 patients who underwent invasive FFR pullback measurement in 112 arteries. First, a multi planar reconstruction (MPR) of the artery is fed to a variational autoencoder to characterize the artery, i.e. through the lumen area and unsupervised artery encodings. Thereafter, a convolutional neural network (CNN) predicts the FFR along the artery. The CNN is supervised by multiple loss functions, notably a loss function inspired by the Earth Mover's Distance (EMD) to predict the correct location of FFR drops and a histogram-based loss to explicitly supervise the slope of the FFR curve. To train and evaluate our model, eight-fold cross-validation was performed. The resulting FFR curves show good agreement with the reference allowing the distinction between diffuse and focal CAD distributions in most cases. Quantitative evaluation yielded a mean absolute difference in the area under the FFR pullback curve (AUPC) of 1.7. The method may pave the way towards fast, accurate, automatic prediction of FFR along the artery from CCTA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge