Stefan K. Piechnik
Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford
SAGCNet: Spatial-Aware Graph Completion Network for Missing Slice Imputation in Population CMR Imaging
Aug 09, 2025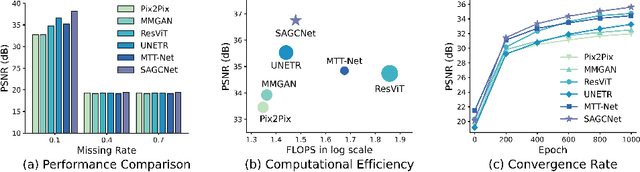
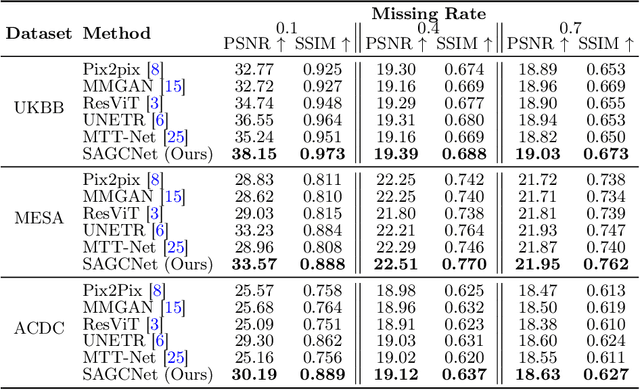
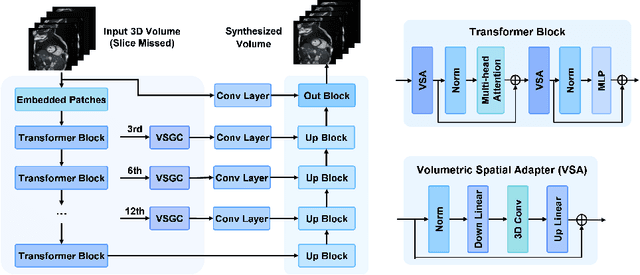

Abstract:Magnetic resonance imaging (MRI) provides detailed soft-tissue characteristics that assist in disease diagnosis and screening. However, the accuracy of clinical practice is often hindered by missing or unusable slices due to various factors. Volumetric MRI synthesis methods have been developed to address this issue by imputing missing slices from available ones. The inherent 3D nature of volumetric MRI data, such as cardiac magnetic resonance (CMR), poses significant challenges for missing slice imputation approaches, including (1) the difficulty of modeling local inter-slice correlations and dependencies of volumetric slices, and (2) the limited exploration of crucial 3D spatial information and global context. In this study, to mitigate these issues, we present Spatial-Aware Graph Completion Network (SAGCNet) to overcome the dependency on complete volumetric data, featuring two main innovations: (1) a volumetric slice graph completion module that incorporates the inter-slice relationships into a graph structure, and (2) a volumetric spatial adapter component that enables our model to effectively capture and utilize various forms of 3D spatial context. Extensive experiments on cardiac MRI datasets demonstrate that SAGCNet is capable of synthesizing absent CMR slices, outperforming competitive state-of-the-art MRI synthesis methods both quantitatively and qualitatively. Notably, our model maintains superior performance even with limited slice data.
Fairness in Cardiac MR Image Analysis: An Investigation of Bias Due to Data Imbalance in Deep Learning Based Segmentation
Jul 01, 2021
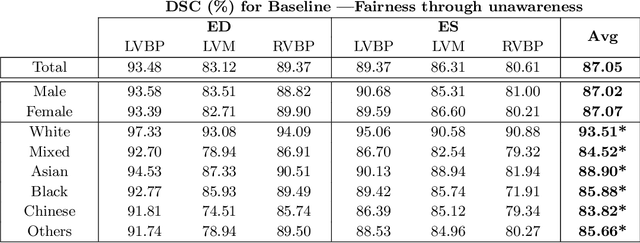

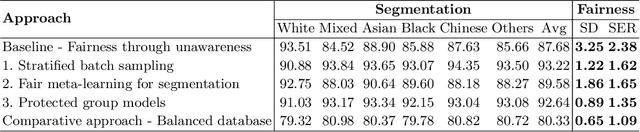
Abstract:The subject of "fairness" in artificial intelligence (AI) refers to assessing AI algorithms for potential bias based on demographic characteristics such as race and gender, and the development of algorithms to address this bias. Most applications to date have been in computer vision, although some work in healthcare has started to emerge. The use of deep learning (DL) in cardiac MR segmentation has led to impressive results in recent years, and such techniques are starting to be translated into clinical practice. However, no work has yet investigated the fairness of such models. In this work, we perform such an analysis for racial/gender groups, focusing on the problem of training data imbalance, using a nnU-Net model trained and evaluated on cine short axis cardiac MR data from the UK Biobank dataset, consisting of 5,903 subjects from 6 different racial groups. We find statistically significant differences in Dice performance between different racial groups. To reduce the racial bias, we investigated three strategies: (1) stratified batch sampling, in which batch sampling is stratified to ensure balance between racial groups; (2) fair meta-learning for segmentation, in which a DL classifier is trained to classify race and jointly optimized with the segmentation model; and (3) protected group models, in which a different segmentation model is trained for each racial group. We also compared the results to the scenario where we have a perfectly balanced database. To assess fairness we used the standard deviation (SD) and skewed error ratio (SER) of the average Dice values. Our results demonstrate that the racial bias results from the use of imbalanced training data, and that all proposed bias mitigation strategies improved fairness, with the best SD and SER resulting from the use of protected group models.
Estimating Uncertainty in Neural Networks for Cardiac MRI Segmentation: A Benchmark Study
Dec 31, 2020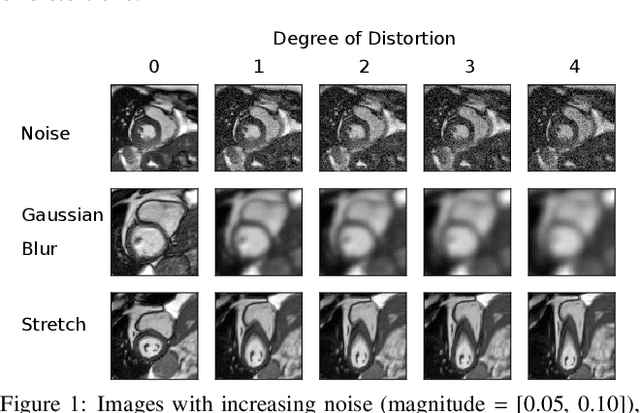
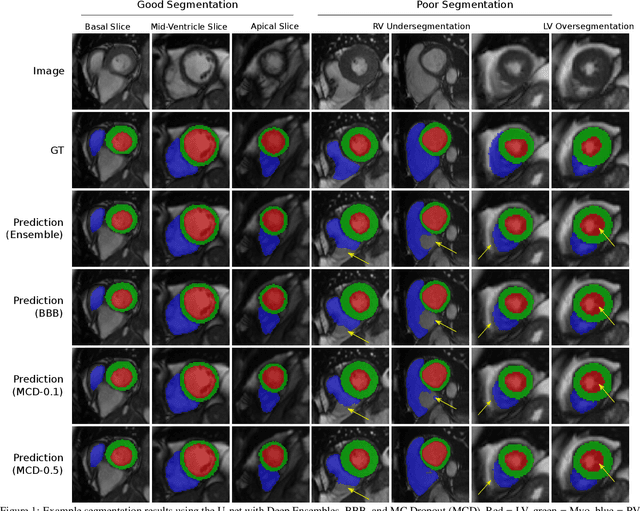
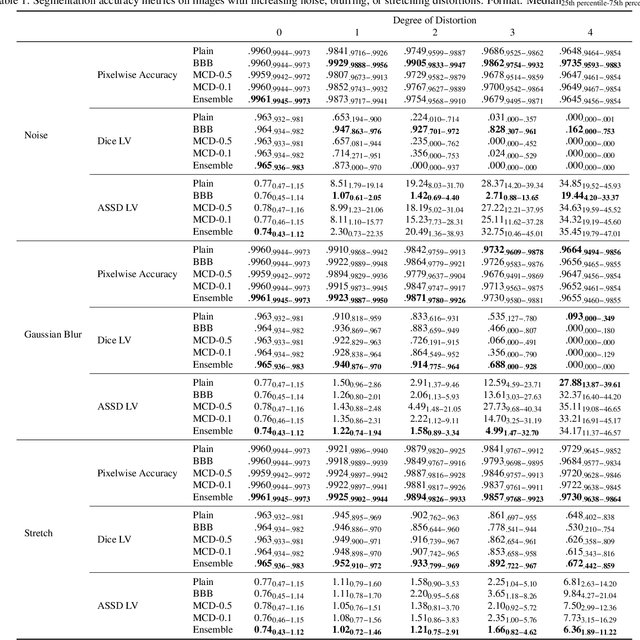
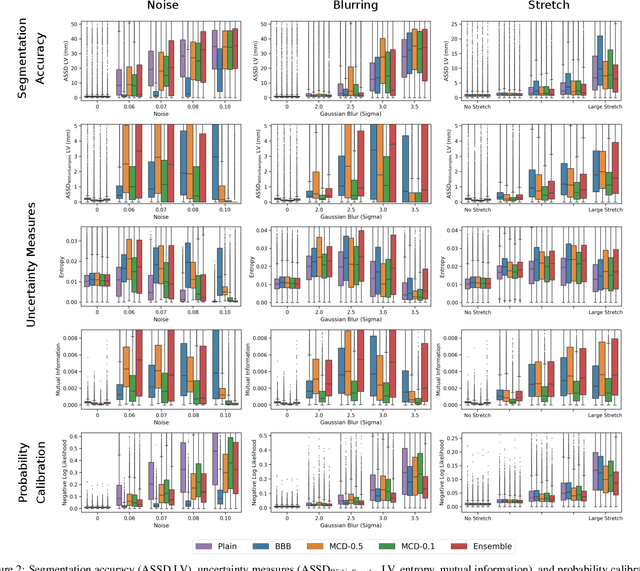
Abstract:Convolutional neural networks (CNNs) have demonstrated promise in automated cardiac magnetic resonance imaging segmentation. However, when using CNNs in a large real world dataset, it is important to quantify segmentation uncertainty in order to know which segmentations could be problematic. In this work, we performed a systematic study of Bayesian and non-Bayesian methods for estimating uncertainty in segmentation neural networks. We evaluated Bayes by Backprop (BBB), Monte Carlo (MC) Dropout, and Deep Ensembles in terms of segmentation accuracy, probability calibration, uncertainty on out-of-distribution images, and segmentation quality control. We tested these algorithms on datasets with various distortions and observed that Deep Ensembles outperformed the other methods except for images with heavy noise distortions. For segmentation quality control, we showed that segmentation uncertainty is correlated with segmentation accuracy. With the incorporation of uncertainty estimates, we were able to reduce the percentage of poor segmentation to 5% by flagging 31% to 48% of the most uncertain images for manual review, substantially lower than random review of the results without using neural network uncertainty.
Fully Automated Myocardial Strain Estimation from CMR Tagged Images using a Deep Learning Framework in the UK Biobank
Apr 15, 2020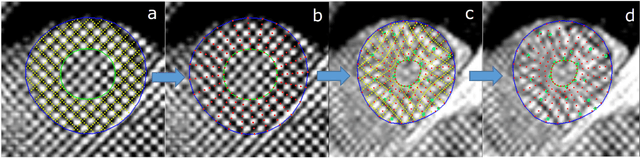
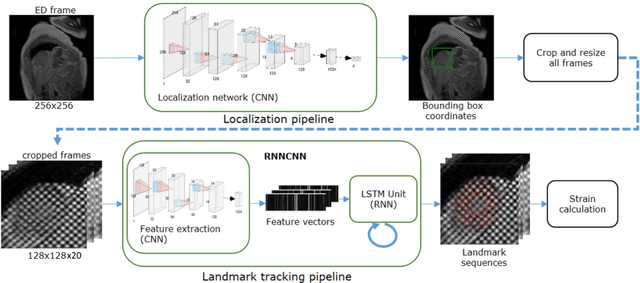
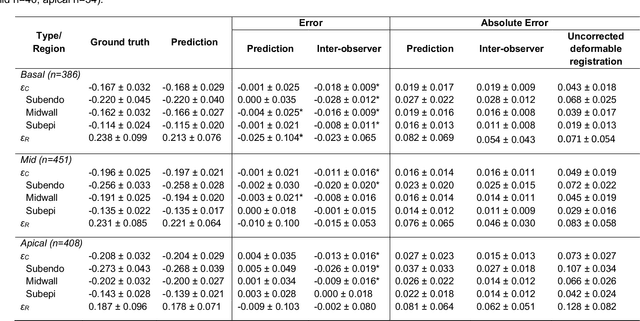
Abstract:Purpose: To demonstrate the feasibility and performance of a fully automated deep learning framework to estimate myocardial strain from short-axis cardiac magnetic resonance tagged images. Methods and Materials: In this retrospective cross-sectional study, 4508 cases from the UK Biobank were split randomly into 3244 training and 812 validation cases, and 452 test cases. Ground truth myocardial landmarks were defined and tracked by manual initialization and correction of deformable image registration using previously validated software with five readers. The fully automatic framework consisted of 1) a convolutional neural network (CNN) for localization, and 2) a combination of a recurrent neural network (RNN) and a CNN to detect and track the myocardial landmarks through the image sequence for each slice. Radial and circumferential strain were then calculated from the motion of the landmarks and averaged on a slice basis. Results: Within the test set, myocardial end-systolic circumferential Green strain errors were -0.001 +/- 0.025, -0.001 +/- 0.021, and 0.004 +/- 0.035 in basal, mid, and apical slices respectively (mean +/- std. dev. of differences between predicted and manual strain). The framework reproduced significant reductions in circumferential strain in diabetics, hypertensives, and participants with previous heart attack. Typical processing time was ~260 frames (~13 slices) per second on an NVIDIA Tesla K40 with 12GB RAM, compared with 6-8 minutes per slice for the manual analysis. Conclusions: The fully automated RNNCNN framework for analysis of myocardial strain enabled unbiased strain evaluation in a high-throughput workflow, with similar ability to distinguish impairment due to diabetes, hypertension, and previous heart attack.
* accepted in Radiology Cardiothoracic Imaging
Joint Motion Estimation and Segmentation from Undersampled Cardiac MR Image
Aug 20, 2019


Abstract:Accelerating the acquisition of magnetic resonance imaging (MRI) is a challenging problem, and many works have been proposed to reconstruct images from undersampled k-space data. However, if the main purpose is to extract certain quantitative measures from the images, perfect reconstructions may not always be necessary as long as the images enable the means of extracting the clinically relevant measures. In this paper, we work on jointly predicting cardiac motion estimation and segmentation directly from undersampled data, which are two important steps in quantitatively assessing cardiac function and diagnosing cardiovascular diseases. In particular, a unified model consisting of both motion estimation branch and segmentation branch is learned by optimising the two tasks simultaneously. Additional corresponding fully-sampled images are incorporated into the network as a parallel sub-network to enhance and guide the learning during the training process. Experimental results using cardiac MR images from 220 subjects show that the proposed model is robust to undersampled data and is capable of predicting results that are close to that from fully-sampled ones, while bypassing the usual image reconstruction stage.
Improving the generalizability of convolutional neural network-based segmentation on CMR images
Jul 03, 2019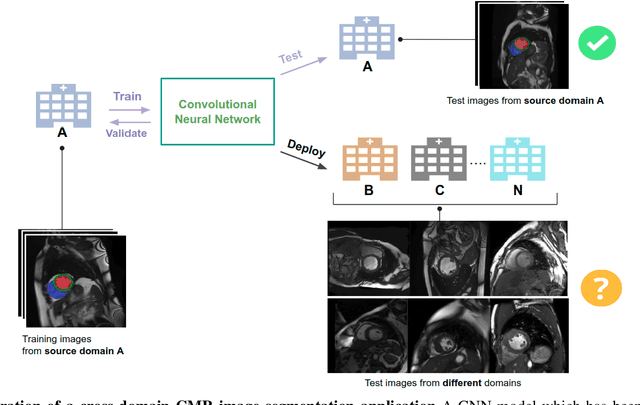
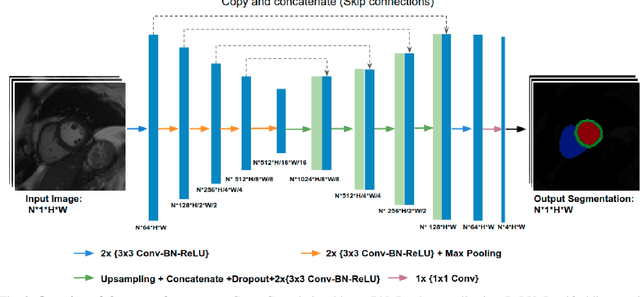
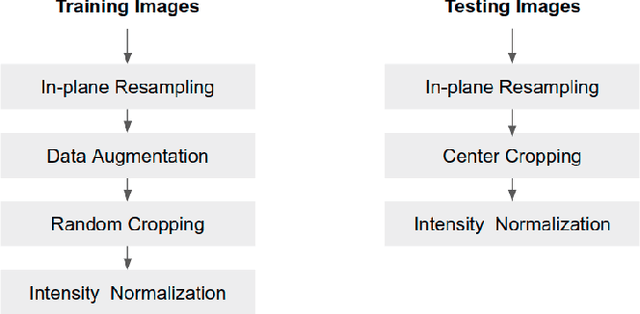
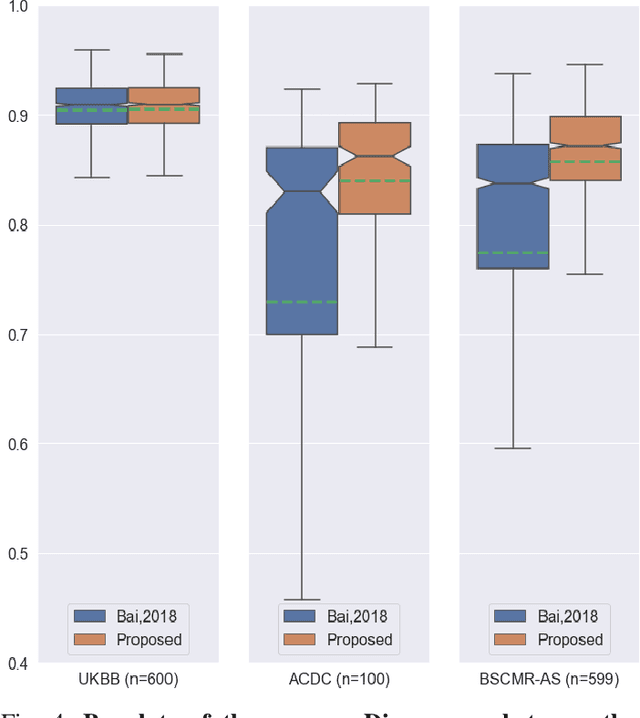
Abstract:Convolutional neural network (CNN) based segmentation methods provide an efficient and automated way for clinicians to assess the structure and function of the heart in cardiac MR images. While CNNs can generally perform the segmentation tasks with high accuracy when training and test images come from the same domain (e.g. same scanner or site), their performance often degrades dramatically on images from different scanners or clinical sites. We propose a simple yet effective way for improving the network generalization ability by carefully designing data normalization and augmentation strategies to accommodate common scenarios in multi-site, multi-scanner clinical imaging data sets. We demonstrate that a neural network trained on a single-site single-scanner dataset from the UK Biobank can be successfully applied to segmenting cardiac MR images across different sites and different scanners without substantial loss of accuracy. Specifically, the method was trained on a large set of 3,975 subjects from the UK Biobank. It was then directly tested on 600 different subjects from the UK Biobank for intra-domain testing and two other sets for cross-domain testing: the ACDC dataset (100 subjects, 1 site, 2 scanners) and the BSCMR-AS dataset (599 subjects, 6 sites, 9 scanners). The proposed method produces promising segmentation results on the UK Biobank test set which are comparable to previously reported values in the literature, while also performing well on cross-domain test sets, achieving a mean Dice metric of 0.90 for the left ventricle, 0.81 for the myocardium and 0.82 for the right ventricle on the ACDC dataset; and 0.89 for the left ventricle, 0.83 for the myocardium on the BSCMR-AS dataset. The proposed method offers a potential solution to improve CNN-based model generalizability for the cross-scanner and cross-site cardiac MR image segmentation task.
3D Cardiac Shape Prediction with Deep Neural Networks: Simultaneous Use of Images and Patient Metadata
Jul 02, 2019
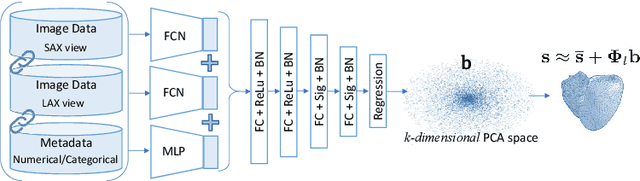
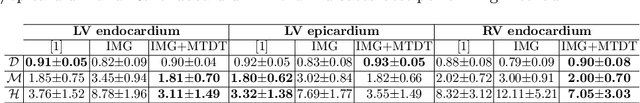
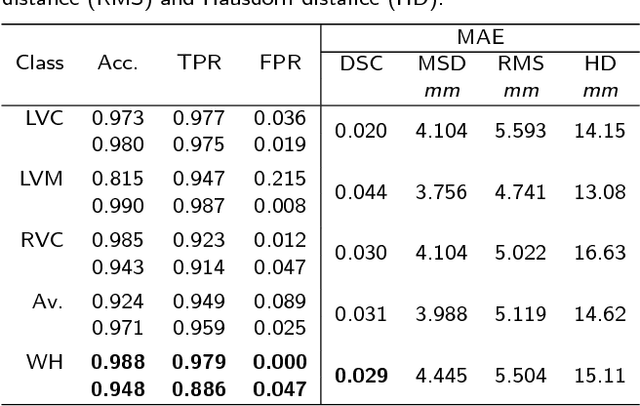
Abstract:Large prospective epidemiological studies acquire cardiovascular magnetic resonance (CMR) images for pre-symptomatic populations and follow these over time. To support this approach, fully automatic large-scale 3D analysis is essential. In this work, we propose a novel deep neural network using both CMR images and patient metadata to directly predict cardiac shape parameters. The proposed method uses the promising ability of statistical shape models to simplify shape complexity and variability together with the advantages of convolutional neural networks for the extraction of solid visual features. To the best of our knowledge, this is the first work that uses such an approach for 3D cardiac shape prediction. We validated our proposed CMR analytics method against a reference cohort containing 500 3D shapes of the cardiac ventricles. Our results show broadly significant agreement with the reference shapes in terms of the estimated volume of the cardiac ventricles, myocardial mass, 3D Dice, and mean and Hausdorff distance.
Automated Quality Control in Image Segmentation: Application to the UK Biobank Cardiac MR Imaging Study
Jan 27, 2019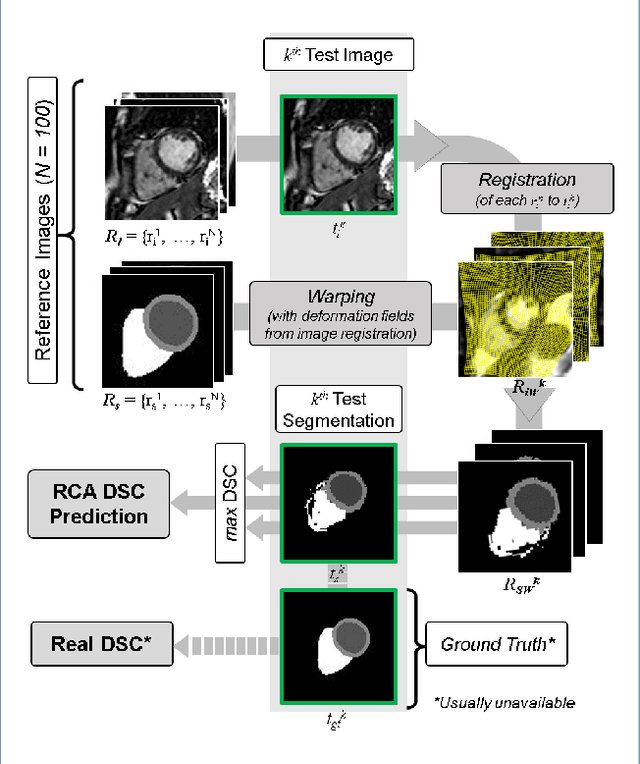
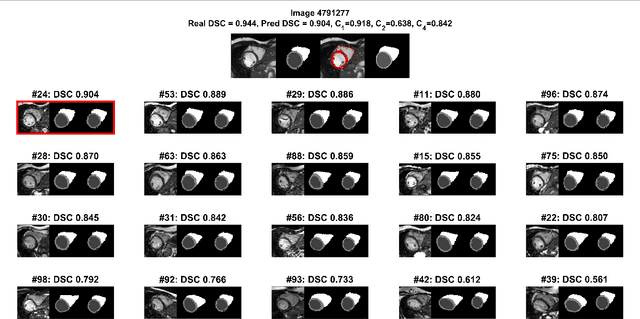
Abstract:Background: The trend towards large-scale studies including population imaging poses new challenges in terms of quality control (QC). This is a particular issue when automatic processing tools, e.g. image segmentation methods, are employed to derive quantitative measures or biomarkers for later analyses. Manual inspection and visual QC of each segmentation isn't feasible at large scale. However, it's important to be able to automatically detect when a segmentation method fails so as to avoid inclusion of wrong measurements into subsequent analyses which could lead to incorrect conclusions. Methods: To overcome this challenge, we explore an approach for predicting segmentation quality based on Reverse Classification Accuracy, which enables us to discriminate between successful and failed segmentations on a per-cases basis. We validate this approach on a new, large-scale manually-annotated set of 4,800 cardiac magnetic resonance scans. We then apply our method to a large cohort of 7,250 cardiac MRI on which we have performed manual QC. Results: We report results used for predicting segmentation quality metrics including Dice Similarity Coefficient (DSC) and surface-distance measures. As initial validation, we present data for 400 scans demonstrating 99% accuracy for classifying low and high quality segmentations using predicted DSC scores. As further validation we show high correlation between real and predicted scores and 95% classification accuracy on 4,800 scans for which manual segmentations were available. We mimic real-world application of the method on 7,250 cardiac MRI where we show good agreement between predicted quality metrics and manual visual QC scores. Conclusions: We show that RCA has the potential for accurate and fully automatic segmentation QC on a per-case basis in the context of large-scale population imaging as in the UK Biobank Imaging Study.
High Throughput Computation of Reference Ranges of Biventricular Cardiac Function on the UK Biobank Population Cohort
Jan 10, 2019



Abstract:The exploitation of large-scale population data has the potential to improve healthcare by discovering and understanding patterns and trends within this data. To enable high throughput analysis of cardiac imaging data automatically, a pipeline should comprise quality monitoring of the input images, segmentation of the cardiac structures, assessment of the segmentation quality, and parsing of cardiac functional indexes. We present a fully automatic, high throughput image parsing workflow for the analysis of cardiac MR images, and test its performance on the UK Biobank (UKB) cardiac dataset. The proposed pipeline is capable of performing end-to-end image processing including: data organisation, image quality assessment, shape model initialisation, segmentation, segmentation quality assessment, and functional parameter computation; all without any user interaction. To the best of our knowledge,this is the first paper tackling the fully automatic 3D analysis of the UKB population study, providing reference ranges for all key cardiovascular functional indexes, from both left and right ventricles of the heart. We tested our workflow on a reference cohort of 800 healthy subjects for which manual delineations, and reference functional indexes exist. Our results show statistically significant agreement between the manually obtained reference indexes, and those automatically computed using our framework.
Automatic Assessment of Full Left Ventricular Coverage in Cardiac Cine Magnetic Resonance Imaging with Fisher-Discriminative 3D CNN
Nov 08, 2018



Abstract:Cardiac magnetic resonance (CMR) images play a growing role in the diagnostic imaging of cardiovascular diseases. Full coverage of the left ventricle (LV), from base to apex, is a basic criterion for CMR image quality and necessary for accurate measurement of cardiac volume and functional assessment. Incomplete coverage of the LV is identified through visual inspection, which is time-consuming and usually done retrospectively in the assessment of large imaging cohorts. This paper proposes a novel automatic method for determining LV coverage from CMR images by using Fisher-discriminative three-dimensional (FD3D) convolutional neural networks (CNNs). In contrast to our previous method employing 2D CNNs, this approach utilizes spatial contextual information in CMR volumes, extracts more representative high-level features and enhances the discriminative capacity of the baseline 2D CNN learning framework, thus achieving superior detection accuracy. A two-stage framework is proposed to identify missing basal and apical slices in measurements of CMR volume. First, the FD3D CNN extracts high-level features from the CMR stacks. These image representations are then used to detect the missing basal and apical slices. Compared to the traditional 3D CNN strategy, the proposed FD3D CNN minimizes within-class scatter and maximizes between-class scatter. We performed extensive experiments to validate the proposed method on more than 5,000 independent volumetric CMR scans from the UK Biobank study, achieving low error rates for missing basal/apical slice detection (4.9\%/4.6\%). The proposed method can also be adopted for assessing LV coverage for other types of CMR image data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge