Edward Ferdian
Deep learning for temporal super-resolution 4D Flow MRI
Jan 15, 2025Abstract:4D Flow Magnetic Resonance Imaging (4D Flow MRI) is a non-invasive technique for volumetric, time-resolved blood flow quantification. However, apparent trade-offs between acquisition time, image noise, and resolution limit clinical applicability. In particular, in regions of highly transient flow, coarse temporal resolution can hinder accurate capture of physiologically relevant flow variations. To overcome these issues, post-processing techniques using deep learning have shown promising results to enhance resolution post-scan using so-called super-resolution networks. However, while super-resolution has been focusing on spatial upsampling, temporal super-resolution remains largely unexplored. The aim of this study was therefore to implement and evaluate a residual network for temporal super-resolution 4D Flow MRI. To achieve this, an existing spatial network (4DFlowNet) was re-designed for temporal upsampling, adapting input dimensions, and optimizing internal layer structures. Training and testing were performed using synthetic 4D Flow MRI data originating from patient-specific in-silico models, as well as using in-vivo datasets. Overall, excellent performance was achieved with input velocities effectively denoised and temporally upsampled, with a mean absolute error (MAE) of 1.0 cm/s in an unseen in-silico setting, outperforming deterministic alternatives (linear interpolation MAE = 2.3 cm/s, sinc interpolation MAE = 2.6 cm/s). Further, the network synthesized high-resolution temporal information from unseen low-resolution in-vivo data, with strong correlation observed at peak flow frames. As such, our results highlight the potential of utilizing data-driven neural networks for temporal super-resolution 4D Flow MRI, enabling high-frame-rate flow quantification without extending acquisition times beyond clinically acceptable limits.
Generalized super-resolution 4D Flow MRI $\unicode{x2013}$ using ensemble learning to extend across the cardiovascular system
Nov 21, 2023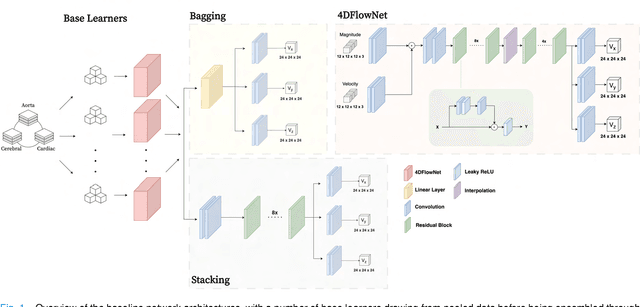
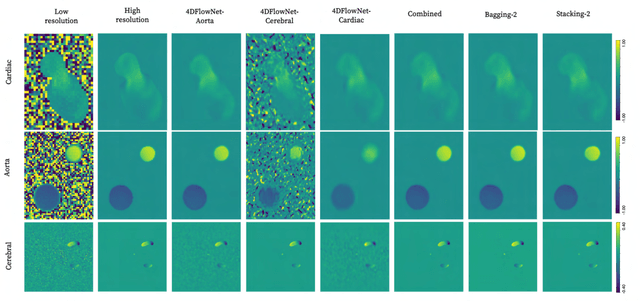
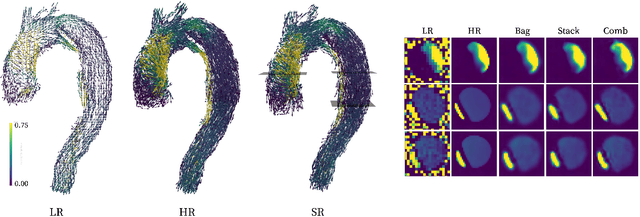
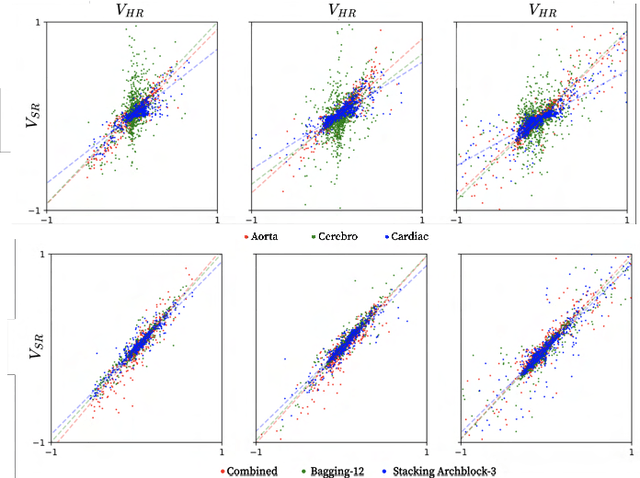
Abstract:4D Flow Magnetic Resonance Imaging (4D Flow MRI) is a non-invasive measurement technique capable of quantifying blood flow across the cardiovascular system. While practical use is limited by spatial resolution and image noise, incorporation of trained super-resolution (SR) networks has potential to enhance image quality post-scan. However, these efforts have predominantly been restricted to narrowly defined cardiovascular domains, with limited exploration of how SR performance extends across the cardiovascular system; a task aggravated by contrasting hemodynamic conditions apparent across the cardiovasculature. The aim of our study was to explore the generalizability of SR 4D Flow MRI using a combination of heterogeneous training sets and dedicated ensemble learning. With synthetic training data generated across three disparate domains (cardiac, aortic, cerebrovascular), varying convolutional base and ensemble learners were evaluated as a function of domain and architecture, quantifying performance on both in-silico and acquired in-vivo data from the same three domains. Results show that both bagging and stacking ensembling enhance SR performance across domains, accurately predicting high-resolution velocities from low-resolution input data in-silico. Likewise, optimized networks successfully recover native resolution velocities from downsampled in-vivo data, as well as show qualitative potential in generating denoised SR-images from clinical level input data. In conclusion, our work presents a viable approach for generalized SR 4D Flow MRI, with ensemble learning extending utility across various clinical areas of interest.
Non-invasive hemodynamic analysis for aortic regurgitation using computational fluid dynamics and deep learning
Nov 23, 2021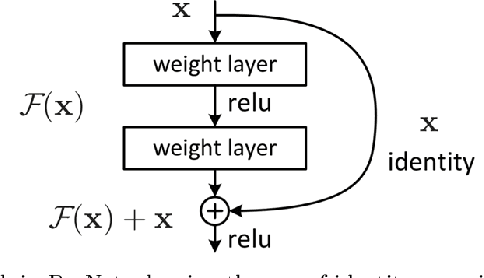
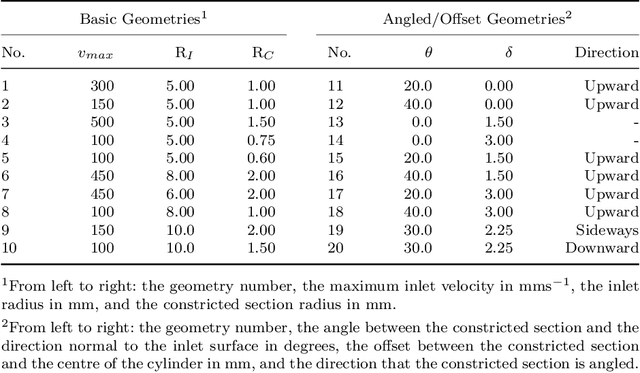
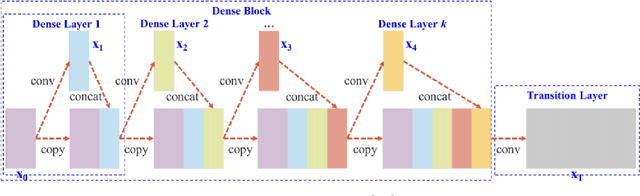
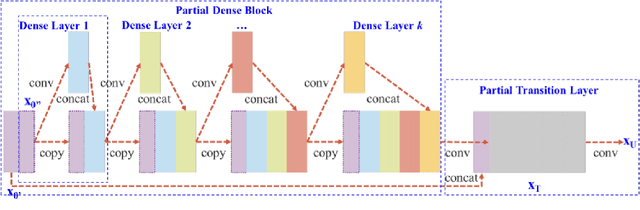
Abstract:Changes in cardiovascular hemodynamics are closely related to the development of aortic regurgitation (AR), a type of valvular heart disease. Pressure gradients derived from blood flows are used to indicate AR onset and evaluate its severity. These metrics can be non-invasively obtained using four-dimensional (4D) flow magnetic resonance imaging (MRI), where accuracy is primarily dependent on spatial resolution. However, insufficient resolution often results from limitations in 4D flow MRI and complex AR hemodynamics. To address this, computational fluid dynamics simulations were transformed into synthetic 4D flow MRI data and used to train a variety of neural networks. These networks generated super resolution, full-field phase images with an upsample factor of 4. Results showed decreased velocity error, high structural similarity scores, and improved learning capabilities from previous work. Further validation was performed on two sets of in-vivo 4D flow MRI data and demonstrated success in de-noising flow images. This approach presents an opportunity to comprehensively analyse AR hemodynamics in a non-invasive manner.
Fully Automated Myocardial Strain Estimation from CMR Tagged Images using a Deep Learning Framework in the UK Biobank
Apr 15, 2020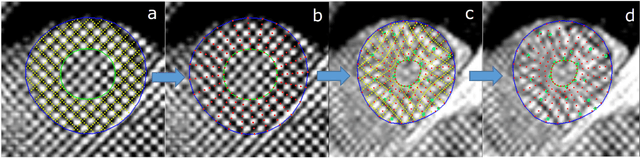
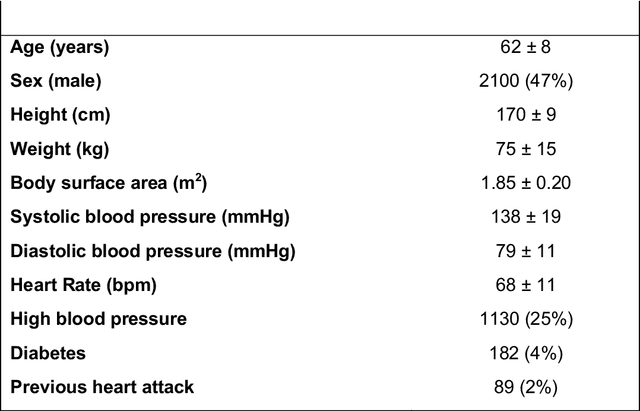
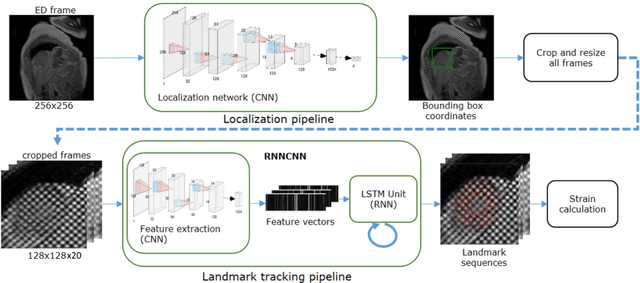
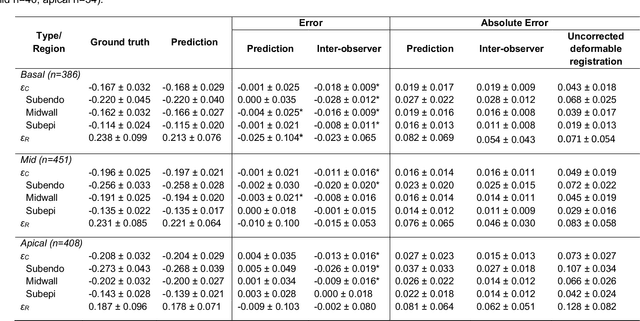
Abstract:Purpose: To demonstrate the feasibility and performance of a fully automated deep learning framework to estimate myocardial strain from short-axis cardiac magnetic resonance tagged images. Methods and Materials: In this retrospective cross-sectional study, 4508 cases from the UK Biobank were split randomly into 3244 training and 812 validation cases, and 452 test cases. Ground truth myocardial landmarks were defined and tracked by manual initialization and correction of deformable image registration using previously validated software with five readers. The fully automatic framework consisted of 1) a convolutional neural network (CNN) for localization, and 2) a combination of a recurrent neural network (RNN) and a CNN to detect and track the myocardial landmarks through the image sequence for each slice. Radial and circumferential strain were then calculated from the motion of the landmarks and averaged on a slice basis. Results: Within the test set, myocardial end-systolic circumferential Green strain errors were -0.001 +/- 0.025, -0.001 +/- 0.021, and 0.004 +/- 0.035 in basal, mid, and apical slices respectively (mean +/- std. dev. of differences between predicted and manual strain). The framework reproduced significant reductions in circumferential strain in diabetics, hypertensives, and participants with previous heart attack. Typical processing time was ~260 frames (~13 slices) per second on an NVIDIA Tesla K40 with 12GB RAM, compared with 6-8 minutes per slice for the manual analysis. Conclusions: The fully automated RNNCNN framework for analysis of myocardial strain enabled unbiased strain evaluation in a high-throughput workflow, with similar ability to distinguish impairment due to diabetes, hypertension, and previous heart attack.
* accepted in Radiology Cardiothoracic Imaging
4DFlowNet: Super-Resolution 4D Flow MRI using Deep Learning and Computational Fluid Dynamics
Apr 15, 2020
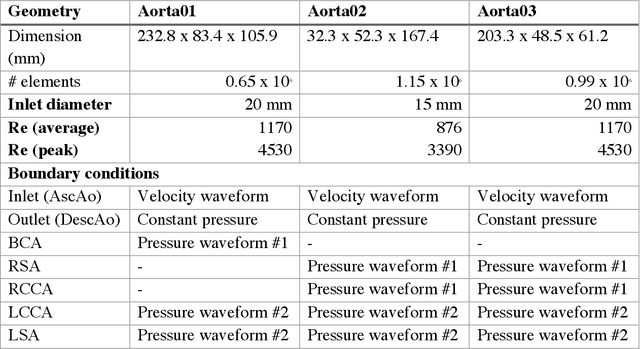

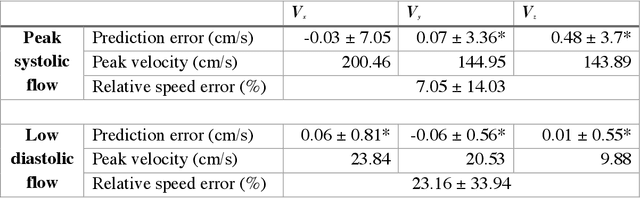
Abstract:4D-flow magnetic resonance imaging (MRI) is an emerging imaging technique where spatiotemporal 3D blood velocity can be captured with full volumetric coverage in a single non-invasive examination. This enables qualitative and quantitative analysis of hemodynamic flow parameters of the heart and great vessels. An increase in the image resolution would provide more accuracy and allow better assessment of the blood flow, especially for patients with abnormal flows. However, this must be balanced with increasing imaging time. The recent success of deep learning in generating super resolution images shows promise for implementation in medical images. We utilized computational fluid dynamics simulations to generate fluid flow simulations and represent them as synthetic 4D flow MRI data. We built our training dataset to mimic actual 4D flow MRI data with its corresponding noise distribution. Our novel 4DFlowNet network was trained on this synthetic 4D flow data and was capable in producing noise-free super resolution 4D flow phase images with upsample factor of 2. We also tested the 4DFlowNet in actual 4D flow MR images of a phantom and normal volunteer data, and demonstrated comparable results with the actual flow rate measurements giving an absolute relative error of 0.6 to 5.8% and 1.1 to 3.8% in the phantom data and normal volunteer data, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge