Shiyi Wang
T-Rex: Task-Adaptive Spatial Representation Extraction for Robotic Manipulation with Vision-Language Models
Jun 24, 2025Abstract:Building a general robotic manipulation system capable of performing a wide variety of tasks in real-world settings is a challenging task. Vision-Language Models (VLMs) have demonstrated remarkable potential in robotic manipulation tasks, primarily due to the extensive world knowledge they gain from large-scale datasets. In this process, Spatial Representations (such as points representing object positions or vectors representing object orientations) act as a bridge between VLMs and real-world scene, effectively grounding the reasoning abilities of VLMs and applying them to specific task scenarios. However, existing VLM-based robotic approaches often adopt a fixed spatial representation extraction scheme for various tasks, resulting in insufficient representational capability or excessive extraction time. In this work, we introduce T-Rex, a Task-Adaptive Framework for Spatial Representation Extraction, which dynamically selects the most appropriate spatial representation extraction scheme for each entity based on specific task requirements. Our key insight is that task complexity determines the types and granularity of spatial representations, and Stronger representational capabilities are typically associated with Higher overall system operation costs. Through comprehensive experiments in real-world robotic environments, we show that our approach delivers significant advantages in spatial understanding, efficiency, and stability without additional training.
AntiGrounding: Lifting Robotic Actions into VLM Representation Space for Decision Making
Jun 14, 2025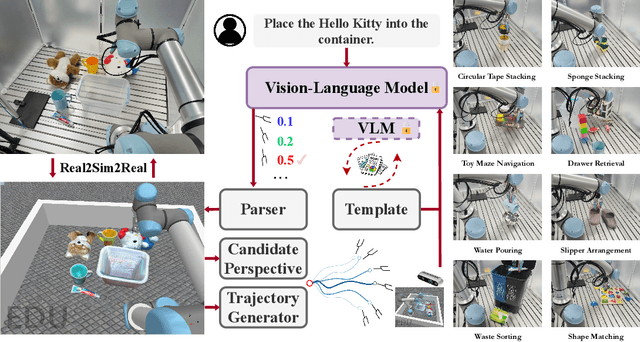
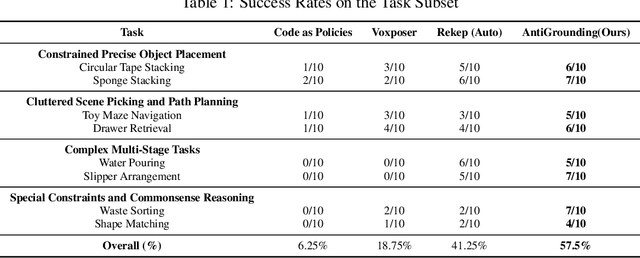
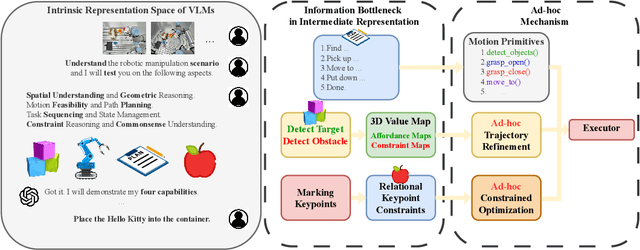

Abstract:Vision-Language Models (VLMs) encode knowledge and reasoning capabilities for robotic manipulation within high-dimensional representation spaces. However, current approaches often project them into compressed intermediate representations, discarding important task-specific information such as fine-grained spatial or semantic details. To address this, we propose AntiGrounding, a new framework that reverses the instruction grounding process. It lifts candidate actions directly into the VLM representation space, renders trajectories from multiple views, and uses structured visual question answering for instruction-based decision making. This enables zero-shot synthesis of optimal closed-loop robot trajectories for new tasks. We also propose an offline policy refinement module that leverages past experience to enhance long-term performance. Experiments in both simulation and real-world environments show that our method outperforms baselines across diverse robotic manipulation tasks.
M2UD: A Multi-model, Multi-scenario, Uneven-terrain Dataset for Ground Robot with Localization and Mapping Evaluation
Mar 16, 2025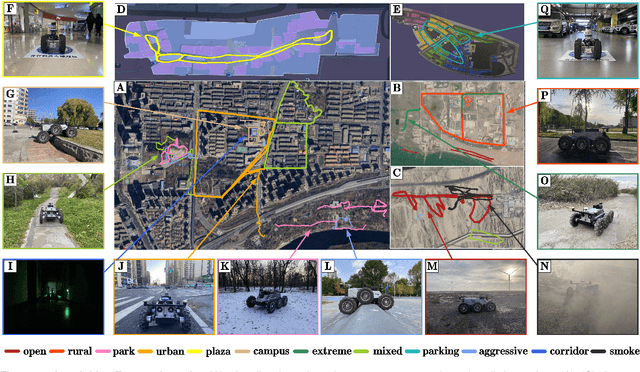
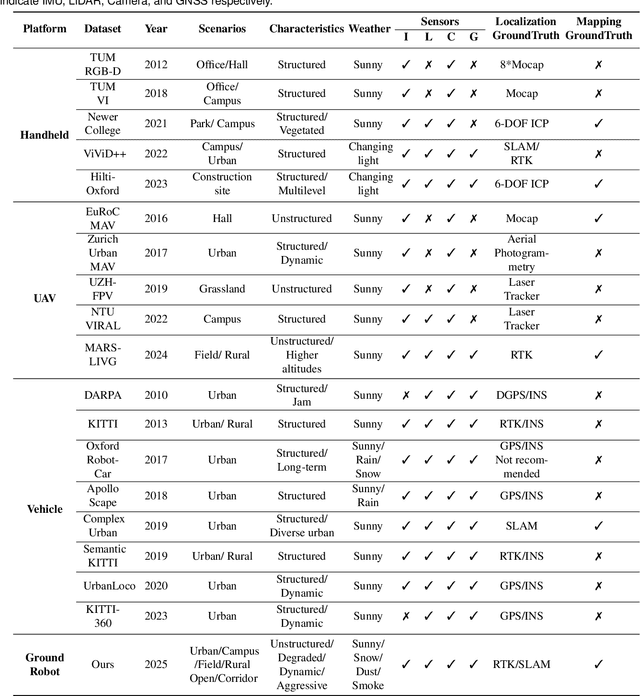
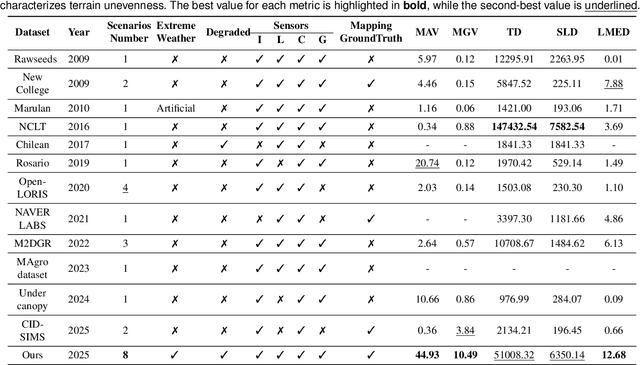
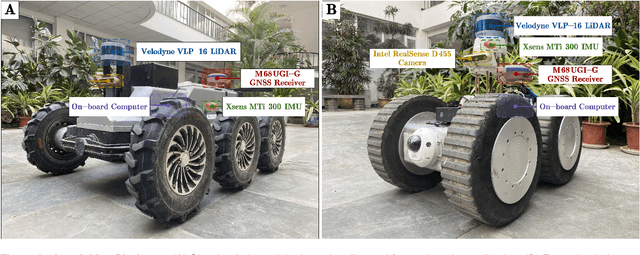
Abstract:Ground robots play a crucial role in inspection, exploration, rescue, and other applications. In recent years, advancements in LiDAR technology have made sensors more accurate, lightweight, and cost-effective. Therefore, researchers increasingly integrate sensors, for SLAM studies, providing robust technical support for ground robots and expanding their application domains. Public datasets are essential for advancing SLAM technology. However, existing datasets for ground robots are typically restricted to flat-terrain motion with 3 DOF and cover only a limited range of scenarios. Although handheld devices and UAV exhibit richer and more aggressive movements, their datasets are predominantly confined to small-scale environments due to endurance limitations. To fill these gap, we introduce M2UD, a multi-modal, multi-scenario, uneven-terrain SLAM dataset for ground robots. This dataset contains a diverse range of highly challenging environments, including cities, open fields, long corridors, and mixed scenarios. Additionally, it presents extreme weather conditions. The aggressive motion and degradation characteristics of this dataset not only pose challenges for testing and evaluating existing SLAM methods but also advance the development of more advanced SLAM algorithms. To benchmark SLAM algorithms, M2UD provides smoothed ground truth localization data obtained via RTK and introduces a novel localization evaluation metric that considers both accuracy and efficiency. Additionally, we utilize a high-precision laser scanner to acquire ground truth maps of two representative scenes, facilitating the development and evaluation of mapping algorithms. We select 12 localization sequences and 2 mapping sequences to evaluate several classical SLAM algorithms, verifying usability of the dataset. To enhance usability, the dataset is accompanied by a suite of development kits.
Will Large Language Models be a Panacea to Autonomous Driving?
Sep 24, 2024



Abstract:Artificial intelligence (AI) plays a crucial role in autonomous driving (AD) research, propelling its development towards intelligence and efficiency. Currently, the development of AD technology follows two main technical paths: modularization and end-to-end. Modularization decompose the driving task into modules such as perception, prediction, planning, and control, and train them separately. Due to the inconsistency of training objectives between modules, the integrated effect suffers from bias. End-to-end attempts to address this issue by utilizing a single model that directly maps from sensor data to control signals. This path has limited learning capabilities in a comprehensive set of features and struggles to handle unpredictable long-tail events and complex urban traffic scenarios. In the face of challenges encountered in both paths, many researchers believe that large language models (LLMs) with powerful reasoning capabilities and extensive knowledge understanding may be the solution, expecting LLMs to provide AD systems with deeper levels of understanding and decision-making capabilities. In light of the challenges faced by both paths, many researchers believe that LLMs, with their powerful reasoning abilities and extensive knowledge, could offer a solution. To understand if LLMs could enhance AD, this paper conducts a thorough analysis of the potential applications of LLMs in AD systems, including exploring their optimization strategies in both modular and end-to-end approaches, with a particular focus on how LLMs can tackle the problems and challenges present in current solutions. Furthermore, we discuss an important question: Can LLM-based artificial general intelligence (AGI) be a key to achieve high-level AD? We further analyze the potential limitations and challenges that LLMs may encounter in promoting the development of AD technology.
Coupling AI and Citizen Science in Creation of Enhanced Training Dataset for Medical Image Segmentation
Sep 04, 2024Abstract:Recent advancements in medical imaging and artificial intelligence (AI) have greatly enhanced diagnostic capabilities, but the development of effective deep learning (DL) models is still constrained by the lack of high-quality annotated datasets. The traditional manual annotation process by medical experts is time- and resource-intensive, limiting the scalability of these datasets. In this work, we introduce a robust and versatile framework that combines AI and crowdsourcing to improve both the quality and quantity of medical image datasets across different modalities. Our approach utilises a user-friendly online platform that enables a diverse group of crowd annotators to label medical images efficiently. By integrating the MedSAM segmentation AI with this platform, we accelerate the annotation process while maintaining expert-level quality through an algorithm that merges crowd-labelled images. Additionally, we employ pix2pixGAN, a generative AI model, to expand the training dataset with synthetic images that capture realistic morphological features. These methods are combined into a cohesive framework designed to produce an enhanced dataset, which can serve as a universal pre-processing pipeline to boost the training of any medical deep learning segmentation model. Our results demonstrate that this framework significantly improves model performance, especially when training data is limited.
Probing Perfection: The Relentless Art of Meddling for Pulmonary Airway Segmentation from HRCT via a Human-AI Collaboration Based Active Learning Method
Jul 03, 2024Abstract:In pulmonary tracheal segmentation, the scarcity of annotated data is a prevalent issue in medical segmentation. Additionally, Deep Learning (DL) methods face challenges: the opacity of 'black box' models and the need for performance enhancement. Our Human-Computer Interaction (HCI) based models (RS_UNet, LC_UNet, UUNet, and WD_UNet) address these challenges by combining diverse query strategies with various DL models. We train four HCI models and repeat these steps: (1) Query Strategy: The HCI models select samples that provide the most additional representative information when labeled in each iteration and identify unlabeled samples with the greatest predictive disparity using Wasserstein Distance, Least Confidence, Entropy Sampling, and Random Sampling. (2) Central line correction: Selected samples are used for expert correction of system-generated tracheal central lines in each training round. (3) Update training dataset: Experts update the training dataset after each DL model's training epoch, enhancing the trustworthiness and performance of the models. (4) Model training: The HCI model is trained using the updated dataset and an enhanced UNet version. Experimental results confirm the effectiveness of these HCI-based approaches, showing that WD-UNet, LC-UNet, UUNet, and RS-UNet achieve comparable or superior performance to state-of-the-art DL models. Notably, WD-UNet achieves this with only 15%-35% of the training data, reducing physician annotation time by 65%-85%.
Fuzzy Attention-based Border Rendering Network for Lung Organ Segmentation
Jun 23, 2024



Abstract:Automatic lung organ segmentation on CT images is crucial for lung disease diagnosis. However, the unlimited voxel values and class imbalance of lung organs can lead to false-negative/positive and leakage issues in advanced methods. Additionally, some slender lung organs are easily lost during the recycled down/up-sample procedure, e.g., bronchioles & arterioles, causing severe discontinuity issue. Inspired by these, this paper introduces an effective lung organ segmentation method called Fuzzy Attention-based Border Rendering (FABR) network. Since fuzzy logic can handle the uncertainty in feature extraction, hence the fusion of deep networks and fuzzy sets should be a viable solution for better performance. Meanwhile, unlike prior top-tier methods that operate on all regular dense points, our FABR depicts lung organ regions as cube-trees, focusing only on recycle-sampled border vulnerable points, rendering the severely discontinuous, false-negative/positive organ regions with a novel Global-Local Cube-tree Fusion (GLCF) module. All experimental results, on four challenging datasets of airway & artery, demonstrate that our method can achieve the favorable performance significantly.
DiffExplainer: Unveiling Black Box Models Via Counterfactual Generation
Jun 21, 2024Abstract:In the field of medical imaging, particularly in tasks related to early disease detection and prognosis, understanding the reasoning behind AI model predictions is imperative for assessing their reliability. Conventional explanation methods encounter challenges in identifying decisive features in medical image classifications, especially when discriminative features are subtle or not immediately evident. To address this limitation, we propose an agent model capable of generating counterfactual images that prompt different decisions when plugged into a black box model. By employing this agent model, we can uncover influential image patterns that impact the black model's final predictions. Through our methodology, we efficiently identify features that influence decisions of the deep black box. We validated our approach in the rigorous domain of medical prognosis tasks, showcasing its efficacy and potential to enhance the reliability of deep learning models in medical image classification compared to existing interpretation methods. The code will be publicly available at https://github.com/ayanglab/DiffExplainer.
Make it more specific: A novel uncertainty based airway segmentation application on 3D U-Net and its variants
Feb 12, 2024Abstract:Each medical segmentation task should be considered with a specific AI algorithm based on its scenario so that the most accurate prediction model can be obtained. The most popular algorithms in medical segmentation, 3D U-Net and its variants, can directly implement the task of lung trachea segmentation, but its failure to consider the special tree-like structure of the trachea suggests that there is much room for improvement in its segmentation accuracy. Therefore, a research gap exists because a great amount of state-of-the-art DL algorithms are vanilla 3D U-Net structures, which do not introduce the various performance-enhancing modules that come with special natural image modality in lung airway segmentation. In this paper, we proposed two different network structures Branch-Level U-Net (B-UNet) and Branch-Level CE-UNet (B-CE-UNet) which are based on U-Net structure and compared the prediction results with the same dataset. Specially, both of the two networks add branch loss and central line loss to learn the feature of fine branch endings of the airways. Uncertainty estimation algorithms are also included to attain confident predictions and thereby, increase the overall trustworthiness of our whole model. In addition, predictions of the lung trachea based on the maximum connectivity rate were calculated and extracted during post-processing for segmentation refinement and pruning.
Hunting imaging biomarkers in pulmonary fibrosis: Benchmarks of the AIIB23 challenge
Dec 21, 2023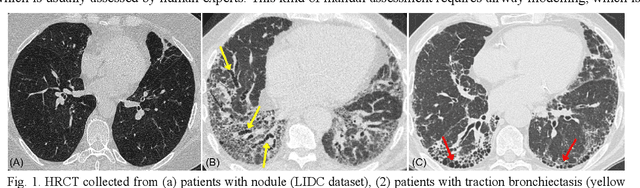
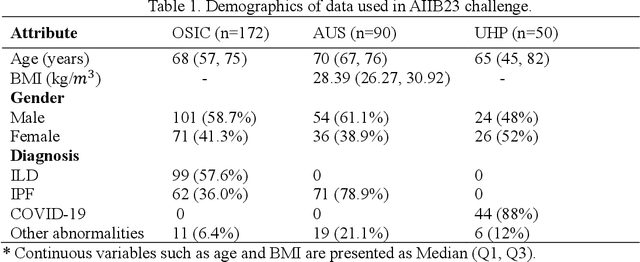
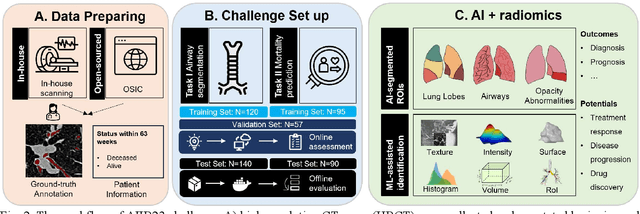
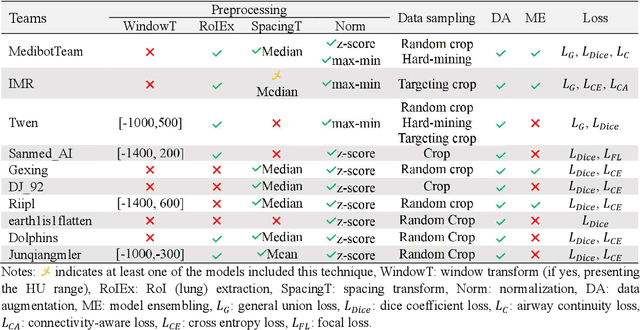
Abstract:Airway-related quantitative imaging biomarkers are crucial for examination, diagnosis, and prognosis in pulmonary diseases. However, the manual delineation of airway trees remains prohibitively time-consuming. While significant efforts have been made towards enhancing airway modelling, current public-available datasets concentrate on lung diseases with moderate morphological variations. The intricate honeycombing patterns present in the lung tissues of fibrotic lung disease patients exacerbate the challenges, often leading to various prediction errors. To address this issue, the 'Airway-Informed Quantitative CT Imaging Biomarker for Fibrotic Lung Disease 2023' (AIIB23) competition was organized in conjunction with the official 2023 International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI). The airway structures were meticulously annotated by three experienced radiologists. Competitors were encouraged to develop automatic airway segmentation models with high robustness and generalization abilities, followed by exploring the most correlated QIB of mortality prediction. A training set of 120 high-resolution computerised tomography (HRCT) scans were publicly released with expert annotations and mortality status. The online validation set incorporated 52 HRCT scans from patients with fibrotic lung disease and the offline test set included 140 cases from fibrosis and COVID-19 patients. The results have shown that the capacity of extracting airway trees from patients with fibrotic lung disease could be enhanced by introducing voxel-wise weighted general union loss and continuity loss. In addition to the competitive image biomarkers for prognosis, a strong airway-derived biomarker (Hazard ratio>1.5, p<0.0001) was revealed for survival prognostication compared with existing clinical measurements, clinician assessment and AI-based biomarkers.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge