Rong Wu
The Agent's First Day: Benchmarking Learning, Exploration, and Scheduling in the Workplace Scenarios
Jan 13, 2026Abstract:The rapid evolution of Multi-modal Large Language Models (MLLMs) has advanced workflow automation; however, existing research mainly targets performance upper bounds in static environments, overlooking robustness for stochastic real-world deployment. We identify three key challenges: dynamic task scheduling, active exploration under uncertainty, and continuous learning from experience. To bridge this gap, we introduce \method{}, a dynamic evaluation environment that simulates a "trainee" agent continuously exploring a novel setting. Unlike traditional benchmarks, \method{} evaluates agents along three dimensions: (1) context-aware scheduling for streaming tasks with varying priorities; (2) prudent information acquisition to reduce hallucination via active exploration; and (3) continuous evolution by distilling generalized strategies from rule-based, dynamically generated tasks. Experiments show that cutting-edge agents have significant deficiencies in dynamic environments, especially in active exploration and continual learning. Our work establishes a framework for assessing agent reliability, shifting evaluation from static tests to realistic, production-oriented scenarios. Our codes are available at https://github.com/KnowledgeXLab/EvoEnv
Learning on the Job: An Experience-Driven Self-Evolving Agent for Long-Horizon Tasks
Oct 09, 2025Abstract:Large Language Models have demonstrated remarkable capabilities across diverse domains, yet significant challenges persist when deploying them as AI agents for real-world long-horizon tasks. Existing LLM agents suffer from a critical limitation: they are test-time static and cannot learn from experience, lacking the ability to accumulate knowledge and continuously improve on the job. To address this challenge, we propose MUSE, a novel agent framework that introduces an experience-driven, self-evolving system centered around a hierarchical Memory Module. MUSE organizes diverse levels of experience and leverages them to plan and execute long-horizon tasks across multiple applications. After each sub-task execution, the agent autonomously reflects on its trajectory, converting the raw trajectory into structured experience and integrating it back into the Memory Module. This mechanism enables the agent to evolve beyond its static pretrained parameters, fostering continuous learning and self-evolution. We evaluate MUSE on the long-horizon productivity benchmark TAC. It achieves new SOTA performance by a significant margin using only a lightweight Gemini-2.5 Flash model. Sufficient Experiments demonstrate that as the agent autonomously accumulates experience, it exhibits increasingly superior task completion capabilities, as well as robust continuous learning and self-evolution capabilities. Moreover, the accumulated experience from MUSE exhibits strong generalization properties, enabling zero-shot improvement on new tasks. MUSE establishes a new paradigm for AI agents capable of real-world productivity task automation.
LeanRAG: Knowledge-Graph-Based Generation with Semantic Aggregation and Hierarchical Retrieval
Aug 14, 2025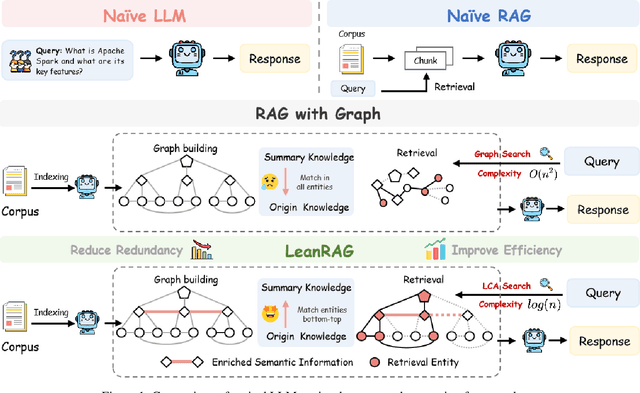
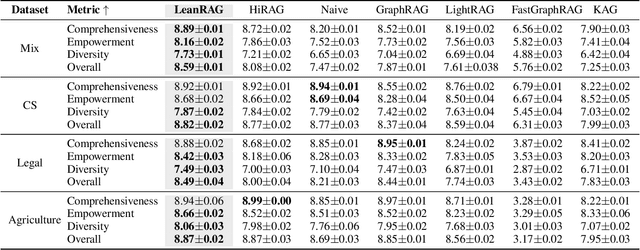
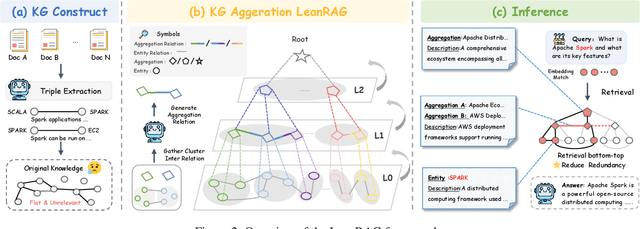
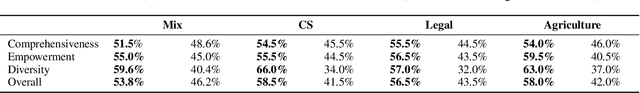
Abstract:Retrieval-Augmented Generation (RAG) plays a crucial role in grounding Large Language Models by leveraging external knowledge, whereas the effectiveness is often compromised by the retrieval of contextually flawed or incomplete information. To address this, knowledge graph-based RAG methods have evolved towards hierarchical structures, organizing knowledge into multi-level summaries. However, these approaches still suffer from two critical, unaddressed challenges: high-level conceptual summaries exist as disconnected ``semantic islands'', lacking the explicit relations needed for cross-community reasoning; and the retrieval process itself remains structurally unaware, often degenerating into an inefficient flat search that fails to exploit the graph's rich topology. To overcome these limitations, we introduce LeanRAG, a framework that features a deeply collaborative design combining knowledge aggregation and retrieval strategies. LeanRAG first employs a novel semantic aggregation algorithm that forms entity clusters and constructs new explicit relations among aggregation-level summaries, creating a fully navigable semantic network. Then, a bottom-up, structure-guided retrieval strategy anchors queries to the most relevant fine-grained entities and then systematically traverses the graph's semantic pathways to gather concise yet contextually comprehensive evidence sets. The LeanRAG can mitigate the substantial overhead associated with path retrieval on graphs and minimizes redundant information retrieval. Extensive experiments on four challenging QA benchmarks with different domains demonstrate that LeanRAG significantly outperforming existing methods in response quality while reducing 46\% retrieval redundancy. Code is available at: https://github.com/RaZzzyz/LeanRAG
Unleashing Diffusion and State Space Models for Medical Image Segmentation
Jun 15, 2025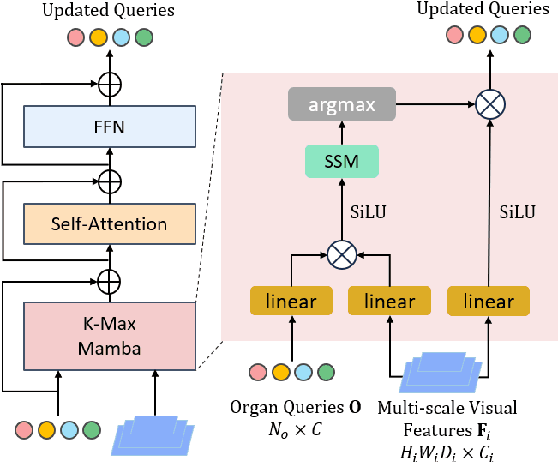
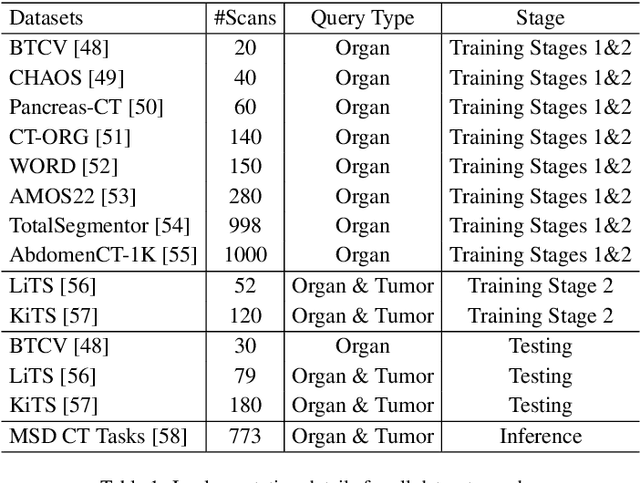
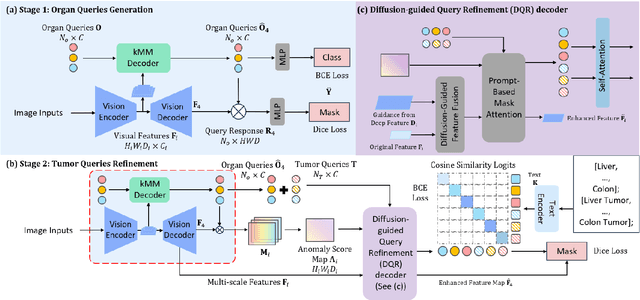
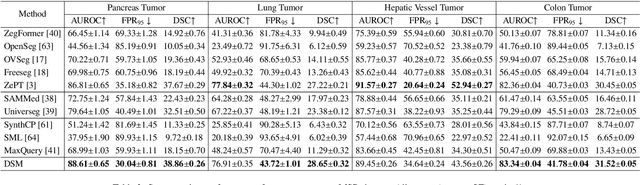
Abstract:Existing segmentation models trained on a single medical imaging dataset often lack robustness when encountering unseen organs or tumors. Developing a robust model capable of identifying rare or novel tumor categories not present during training is crucial for advancing medical imaging applications. We propose DSM, a novel framework that leverages diffusion and state space models to segment unseen tumor categories beyond the training data. DSM utilizes two sets of object queries trained within modified attention decoders to enhance classification accuracy. Initially, the model learns organ queries using an object-aware feature grouping strategy to capture organ-level visual features. It then refines tumor queries by focusing on diffusion-based visual prompts, enabling precise segmentation of previously unseen tumors. Furthermore, we incorporate diffusion-guided feature fusion to improve semantic segmentation performance. By integrating CLIP text embeddings, DSM captures category-sensitive classes to improve linguistic transfer knowledge, thereby enhancing the model's robustness across diverse scenarios and multi-label tasks. Extensive experiments demonstrate the superior performance of DSM in various tumor segmentation tasks. Code is available at https://github.com/Rows21/KMax-Mamba.
O$^2$-Searcher: A Searching-based Agent Model for Open-Domain Open-Ended Question Answering
May 22, 2025Abstract:Large Language Models (LLMs), despite their advancements, are fundamentally limited by their static parametric knowledge, hindering performance on tasks requiring open-domain up-to-date information. While enabling LLMs to interact with external knowledge environments is a promising solution, current efforts primarily address closed-end problems. Open-ended questions, which characterized by lacking a standard answer or providing non-unique and diverse answers, remain underexplored. To bridge this gap, we present O$^2$-Searcher, a novel search agent leveraging reinforcement learning to effectively tackle both open-ended and closed-ended questions in the open domain. O$^2$-Searcher leverages an efficient, locally simulated search environment for dynamic knowledge acquisition, effectively decoupling the external world knowledge from model's sophisticated reasoning processes. It employs a unified training mechanism with meticulously designed reward functions, enabling the agent to identify problem types and adapt different answer generation strategies. Furthermore, to evaluate performance on complex open-ended tasks, we construct O$^2$-QA, a high-quality benchmark featuring 300 manually curated, multi-domain open-ended questions with associated web page caches. Extensive experiments show that O$^2$-Searcher, using only a 3B model, significantly surpasses leading LLM agents on O$^2$-QA. It also achieves SOTA results on various closed-ended QA benchmarks against similarly-sized models, while performing on par with much larger ones.
Nonlinear Sparse Generalized Canonical Correlation Analysis for Multi-view High-dimensional Data
Feb 26, 2025Abstract:Motivation: Biomedical studies increasingly produce multi-view high-dimensional datasets (e.g., multi-omics) that demand integrative analysis. Existing canonical correlation analysis (CCA) and generalized CCA methods address at most two of the following three key aspects simultaneously: (i) nonlinear dependence, (ii) sparsity for variable selection, and (iii) generalization to more than two data views. There is a pressing need for CCA methods that integrate all three aspects to effectively analyze multi-view high-dimensional data. Results: We propose three nonlinear, sparse, generalized CCA methods, HSIC-SGCCA, SA-KGCCA, and TS-KGCCA, for variable selection in multi-view high-dimensional data. These methods extend existing SCCA-HSIC, SA-KCCA, and TS-KCCA from two-view to multi-view settings. While SA-KGCCA and TS-KGCCA yield multi-convex optimization problems solved via block coordinate descent, HSIC-SGCCA introduces a necessary unit-variance constraint previously ignored in SCCA-HSIC, resulting in a nonconvex, non-multiconvex problem. We efficiently address this challenge by integrating the block prox-linear method with the linearized alternating direction method of multipliers. Simulations and TCGA-BRCA data analysis demonstrate that HSIC-SGCCA outperforms competing methods in multi-view variable selection.
Semi-supervised Medical Image Segmentation via Query Distribution Consistency
Nov 21, 2023Abstract:Semi-supervised learning is increasingly popular in medical image segmentation due to its ability to leverage large amounts of unlabeled data to extract additional information. However, most existing semi-supervised segmentation methods focus only on extracting information from unlabeled data. In this paper, we propose a novel Dual KMax UX-Net framework that leverages labeled data to guide the extraction of information from unlabeled data. Our approach is based on a mutual learning strategy that incorporates two modules: 3D UX-Net as our backbone meta-architecture and KMax decoder to enhance the segmentation performance. Extensive experiments on the Atrial Segmentation Challenge dataset have shown that our method can significantly improve performance by merging unlabeled data. Meanwhile, our framework outperforms state-of-the-art semi-supervised learning methods on 10\% and 20\% labeled settings. Code located at: https://github.com/Rows21/DK-UXNet.
Hybrid Representation Learning for Cognitive Diagnosis in Late-Life Depression Over 5 Years with Structural MRI
Dec 24, 2022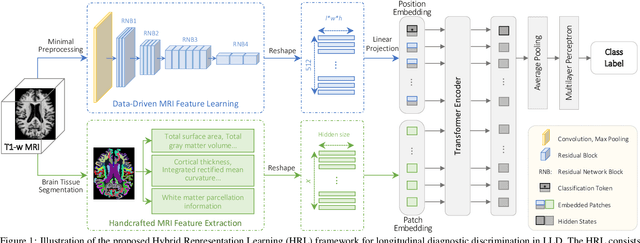
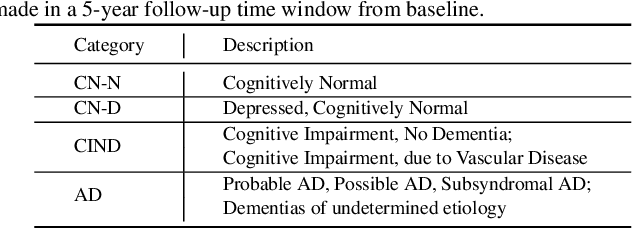
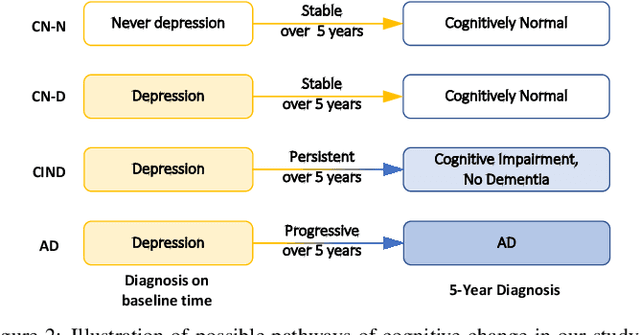
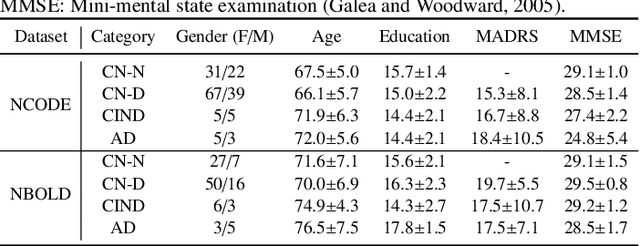
Abstract:Late-life depression (LLD) is a highly prevalent mood disorder occurring in older adults and is frequently accompanied by cognitive impairment (CI). Studies have shown that LLD may increase the risk of Alzheimer's disease (AD). However, the heterogeneity of presentation of geriatric depression suggests that multiple biological mechanisms may underlie it. Current biological research on LLD progression incorporates machine learning that combines neuroimaging data with clinical observations. There are few studies on incident cognitive diagnostic outcomes in LLD based on structural MRI (sMRI). In this paper, we describe the development of a hybrid representation learning (HRL) framework for predicting cognitive diagnosis over 5 years based on T1-weighted sMRI data. Specifically, we first extract prediction-oriented MRI features via a deep neural network, and then integrate them with handcrafted MRI features via a Transformer encoder for cognitive diagnosis prediction. Two tasks are investigated in this work, including (1) identifying cognitively normal subjects with LLD and never-depressed older healthy subjects, and (2) identifying LLD subjects who developed CI (or even AD) and those who stayed cognitively normal over five years. To the best of our knowledge, this is among the first attempts to study the complex heterogeneous progression of LLD based on task-oriented and handcrafted MRI features. We validate the proposed HRL on 294 subjects with T1-weighted MRIs from two clinically harmonized studies. Experimental results suggest that the HRL outperforms several classical machine learning and state-of-the-art deep learning methods in LLD identification and prediction tasks.
Enhancing Non-mass Breast Ultrasound Cancer Classification With Knowledge Transfer
Apr 18, 2022

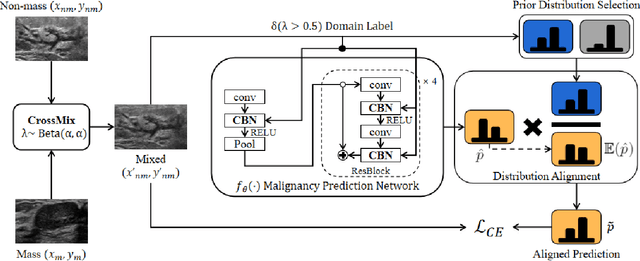
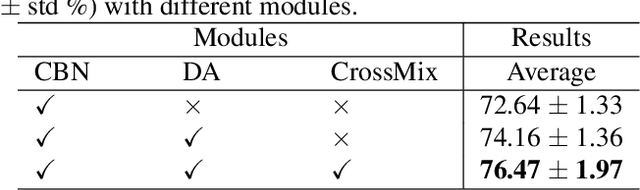
Abstract:Much progress has been made in the deep neural network (DNN) based diagnosis of mass lesions breast ultrasound (BUS) images. However, the non-mass lesion is less investigated because of the limited data. Based on the insight that mass data is sufficient and shares the same knowledge structure with non-mass data of identifying the malignancy of a lesion based on the ultrasound image, we propose a novel transfer learning framework to enhance the generalizability of the DNN model for non-mass BUS with the help of mass BUS. Specifically, we train a shared DNN with combined non-mass and mass data. With the prior of different marginal distributions in input and output space, we employ two domain alignment strategies in the proposed transfer learning framework with the insight of capturing domain-specific distribution to address the issue of domain shift. Moreover, we propose a cross-domain semantic-preserve data generation module called CrossMix to recover the missing distribution between non-mass and mass data that is not presented in training data. Experimental results on an in-house dataset demonstrate that the DNN model trained with combined data by our framework achieves a 10% improvement in AUC on the malignancy prediction task of non-mass BUS compared to training directly on non-mass data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge