Minhui Yu
RelA-Diffusion: Relativistic Adversarial Diffusion for Multi-Tracer PET Synthesis from Multi-Sequence MRI
Feb 24, 2026Abstract:Multi-tracer positron emission tomography (PET) provides critical insights into diverse neuropathological processes such as tau accumulation, neuroinflammation, and $β$-amyloid deposition in the brain, making it indispensable for comprehensive neurological assessment. However, routine acquisition of multi-tracer PET is limited by high costs, radiation exposure, and restricted tracer availability. Recent efforts have explored deep learning approaches for synthesizing PET images from structural MRI. While some methods rely solely on T1-weighted MRI, others incorporate additional sequences such as T2-FLAIR to improve pathological sensitivity. However, existing methods often struggle to capture fine-grained anatomical and pathological details, resulting in artifacts and unrealistic outputs. To this end, we propose RelA-Diffusion, a Relativistic Adversarial Diffusion framework for multi-tracer PET synthesis from multi-sequence MRI. By leveraging both T1-weighted and T2-FLAIR scans as complementary inputs, RelA-Diffusion captures richer structural information to guide image generation. To improve synthesis fidelity, we introduce a gradient-penalized relativistic adversarial loss to the intermediate clean predictions of the diffusion model. This loss compares real and generated images in a relative manner, encouraging the synthesis of more realistic local structures. Both the relativistic formulation and the gradient penalty contribute to stabilizing the training, while adversarial feedback at each diffusion timestep enables consistent refinement throughout the generation process. Extensive experiments on two datasets demonstrate that RelA-Diffusion outperforms existing methods in both visual fidelity and quantitative metrics, highlighting its potential for accurate synthesis of multi-tracer PET.
Unpaired Volumetric Harmonization of Brain MRI with Conditional Latent Diffusion
Aug 18, 2024

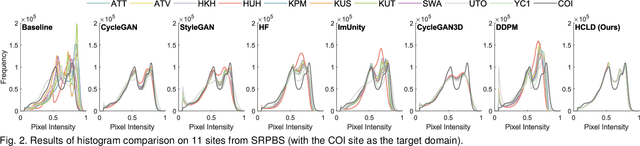

Abstract:Multi-site structural MRI is increasingly used in neuroimaging studies to diversify subject cohorts. However, combining MR images acquired from various sites/centers may introduce site-related non-biological variations. Retrospective image harmonization helps address this issue, but current methods usually perform harmonization on pre-extracted hand-crafted radiomic features, limiting downstream applicability. Several image-level approaches focus on 2D slices, disregarding inherent volumetric information, leading to suboptimal outcomes. To this end, we propose a novel 3D MRI Harmonization framework through Conditional Latent Diffusion (HCLD) by explicitly considering image style and brain anatomy. It comprises a generalizable 3D autoencoder that encodes and decodes MRIs through a 4D latent space, and a conditional latent diffusion model that learns the latent distribution and generates harmonized MRIs with anatomical information from source MRIs while conditioned on target image style. This enables efficient volume-level MRI harmonization through latent style translation, without requiring paired images from target and source domains during training. The HCLD is trained and evaluated on 4,158 T1-weighted brain MRIs from three datasets in three tasks, assessing its ability to remove site-related variations while retaining essential biological features. Qualitative and quantitative experiments suggest the effectiveness of HCLD over several state-of-the-arts
Functional Imaging Constrained Diffusion for Brain PET Synthesis from Structural MRI
May 03, 2024



Abstract:Magnetic resonance imaging (MRI) and positron emission tomography (PET) are increasingly used in multimodal analysis of neurodegenerative disorders. While MRI is broadly utilized in clinical settings, PET is less accessible. Many studies have attempted to use deep generative models to synthesize PET from MRI scans. However, they often suffer from unstable training and inadequately preserve brain functional information conveyed by PET. To this end, we propose a functional imaging constrained diffusion (FICD) framework for 3D brain PET image synthesis with paired structural MRI as input condition, through a new constrained diffusion model (CDM). The FICD introduces noise to PET and then progressively removes it with CDM, ensuring high output fidelity throughout a stable training phase. The CDM learns to predict denoised PET with a functional imaging constraint introduced to ensure voxel-wise alignment between each denoised PET and its ground truth. Quantitative and qualitative analyses conducted on 293 subjects with paired T1-weighted MRI and 18F-fluorodeoxyglucose (FDG)-PET scans suggest that FICD achieves superior performance in generating FDG-PET data compared to state-of-the-art methods. We further validate the effectiveness of the proposed FICD on data from a total of 1,262 subjects through three downstream tasks, with experimental results suggesting its utility and generalizability.
Hybrid Representation Learning for Cognitive Diagnosis in Late-Life Depression Over 5 Years with Structural MRI
Dec 24, 2022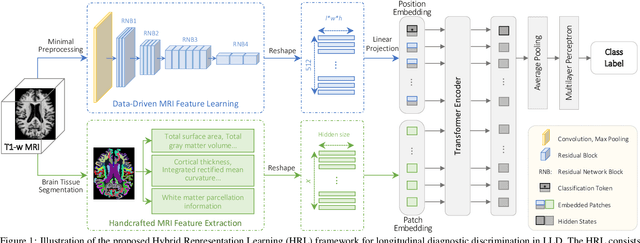
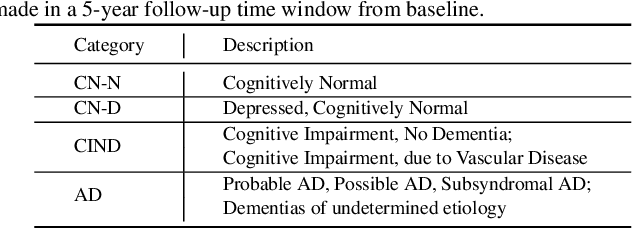
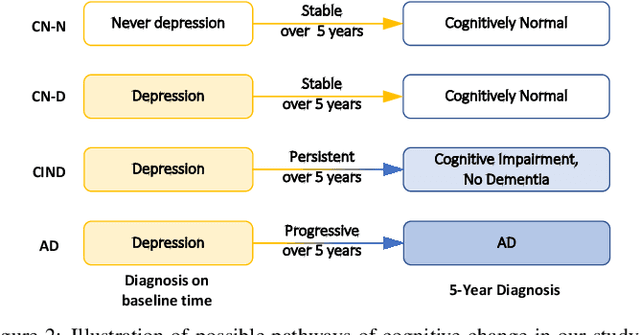
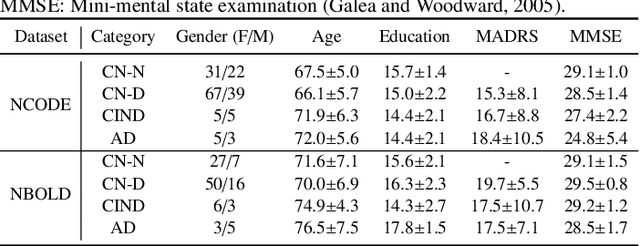
Abstract:Late-life depression (LLD) is a highly prevalent mood disorder occurring in older adults and is frequently accompanied by cognitive impairment (CI). Studies have shown that LLD may increase the risk of Alzheimer's disease (AD). However, the heterogeneity of presentation of geriatric depression suggests that multiple biological mechanisms may underlie it. Current biological research on LLD progression incorporates machine learning that combines neuroimaging data with clinical observations. There are few studies on incident cognitive diagnostic outcomes in LLD based on structural MRI (sMRI). In this paper, we describe the development of a hybrid representation learning (HRL) framework for predicting cognitive diagnosis over 5 years based on T1-weighted sMRI data. Specifically, we first extract prediction-oriented MRI features via a deep neural network, and then integrate them with handcrafted MRI features via a Transformer encoder for cognitive diagnosis prediction. Two tasks are investigated in this work, including (1) identifying cognitively normal subjects with LLD and never-depressed older healthy subjects, and (2) identifying LLD subjects who developed CI (or even AD) and those who stayed cognitively normal over five years. To the best of our knowledge, this is among the first attempts to study the complex heterogeneous progression of LLD based on task-oriented and handcrafted MRI features. We validate the proposed HRL on 294 subjects with T1-weighted MRIs from two clinically harmonized studies. Experimental results suggest that the HRL outperforms several classical machine learning and state-of-the-art deep learning methods in LLD identification and prediction tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge