Razvan Marinescu
Holographic generative flows with AdS/CFT
Jan 29, 2026Abstract:We present a framework for generative machine learning that leverages the holographic principle of quantum gravity, or to be more precise its manifestation as the anti-de Sitter/conformal field theory (AdS/CFT) correspondence, with techniques for deep learning and transport theory. Our proposal is to represent the flow of data from a base distribution to some learned distribution using the bulk-to-boundary mapping of scalar fields in AdS. In the language of machine learning, we are representing and augmenting the flow-matching algorithm with AdS physics. Using a checkerboard toy dataset and MNIST, we find that our model achieves faster and higher quality convergence than comparable physics-free flow-matching models. Our method provides a physically interpretable version of flow matching. More broadly, it establishes the utility of AdS physics and geometry in the development of novel paradigms in generative modeling.
Automatic Replication of LLM Mistakes in Medical Conversations
Dec 24, 2025Abstract:Large language models (LLMs) are increasingly evaluated in clinical settings using multi-dimensional rubrics which quantify reasoning quality, safety, and patient-centeredness. Yet, replicating specific mistakes in other LLM models is not straightforward and often requires manual effort. We introduce MedMistake, an automatic pipeline that extracts mistakes LLMs make in patient-doctor conversations and converts them into a benchmark of single-shot QA pairs. Our pipeline (1) creates complex, conversational data between an LLM patient and LLM doctor, (2) runs an evaluation with a committee of 2 LLM judges across a variety of dimensions and (3) creates simplified single-shot QA scenarios from those mistakes. We release MedMistake-All, a dataset of 3,390 single-shot QA pairs where GPT-5 and Gemini 2.5 Pro are currently failing to answer correctly, as judged by two LLM judges. We used medical experts to validate a subset of 211/3390 questions (MedMistake-Bench), which we used to run a final evaluation of 12 frontier LLMs: Claude Opus 4.5, Claude Sonnet 4.5, DeepSeek-Chat, Gemini 2.5 Pro, Gemini 3 Pro, GPT-4o, GPT-5, GPT-5.1, GPT-5.2, Grok 4, Grok 4.1, Mistral Large. We found that GPT models, Claude and Grok obtained the best performance on MedMistake-Bench. We release both the doctor-validated benchmark (MedMistake-Bench), as well as the full dataset (MedMistake-All) at https://huggingface.co/datasets/TheLumos/MedicalMistakeBenchmark.
A Women's Health Benchmark for Large Language Models
Dec 18, 2025Abstract:As large language models (LLMs) become primary sources of health information for millions, their accuracy in women's health remains critically unexamined. We introduce the Women's Health Benchmark (WHB), the first benchmark evaluating LLM performance specifically in women's health. Our benchmark comprises 96 rigorously validated model stumps covering five medical specialties (obstetrics and gynecology, emergency medicine, primary care, oncology, and neurology), three query types (patient query, clinician query, and evidence/policy query), and eight error types (dosage/medication errors, missing critical information, outdated guidelines/treatment recommendations, incorrect treatment advice, incorrect factual information, missing/incorrect differential diagnosis, missed urgency, and inappropriate recommendations). We evaluated 13 state-of-the-art LLMs and revealed alarming gaps: current models show approximately 60\% failure rates on the women's health benchmark, with performance varying dramatically across specialties and error types. Notably, models universally struggle with "missed urgency" indicators, while newer models like GPT-5 show significant improvements in avoiding inappropriate recommendations. Our findings underscore that AI chatbots are not yet fully able of providing reliable advice in women's health.
TICA-Based Free Energy Matching for Machine-Learned Molecular Dynamics
Sep 18, 2025Abstract:Molecular dynamics (MD) simulations provide atomistic insight into biomolecular systems but are often limited by high computational costs required to access long timescales. Coarse-grained machine learning models offer a promising avenue for accelerating sampling, yet conventional force matching approaches often fail to capture the full thermodynamic landscape as fitting a model on the gradient may not fit the absolute differences between low-energy conformational states. In this work, we incorporate a complementary energy matching term into the loss function. We evaluate our framework on the Chignolin protein using the CGSchNet model, systematically varying the weight of the energy loss term. While energy matching did not yield statistically significant improvements in accuracy, it revealed distinct tendencies in how models generalize the free energy surface. Our results suggest future opportunities to enhance coarse-grained modeling through improved energy estimation techniques and multi-modal loss formulations.
ReMiDi: Reconstruction of Microstructure Using a Differentiable Diffusion MRI Simulator
Feb 04, 2025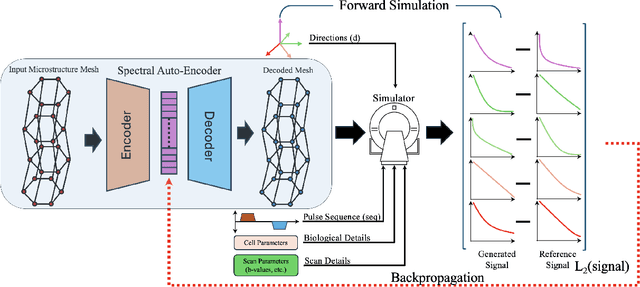
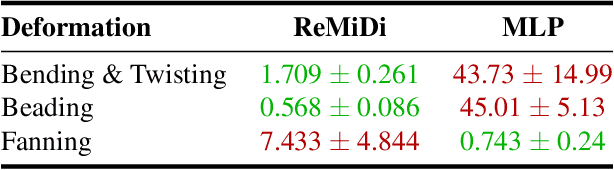
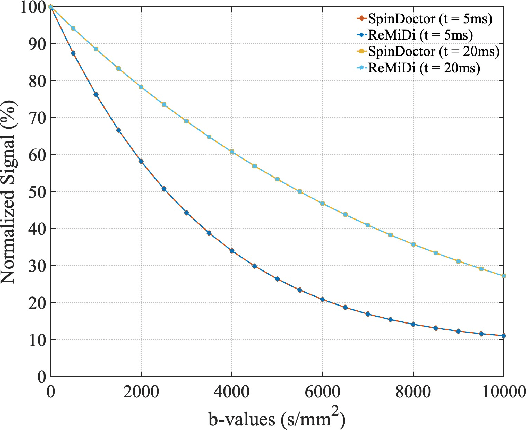
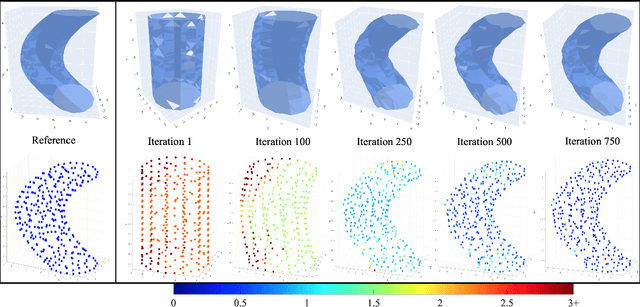
Abstract:We propose ReMiDi, a novel method for inferring neuronal microstructure as arbitrary 3D meshes using a differentiable diffusion Magnetic Resonance Imaging (dMRI) simulator. We first implemented in PyTorch a differentiable dMRI simulator that simulates the forward diffusion process using a finite-element method on an input 3D microstructure mesh. To achieve significantly faster simulations, we solve the differential equation semi-analytically using a matrix formalism approach. Given a reference dMRI signal $S_{ref}$, we use the differentiable simulator to iteratively update the input mesh such that it matches $S_{ref}$ using gradient-based learning. Since directly optimizing the 3D coordinates of the vertices is challenging, particularly due to ill-posedness of the inverse problem, we instead optimize a lower-dimensional latent space representation of the mesh. The mesh is first encoded into spectral coefficients, which are further encoded into a latent $\textbf{z}$ using an auto-encoder, and are then decoded back into the true mesh. We present an end-to-end differentiable pipeline that simulates signals that can be tuned to match a reference signal by iteratively updating the latent representation $\textbf{z}$. We demonstrate the ability to reconstruct microstructures of arbitrary shapes represented by finite-element meshes, with a focus on axonal geometries found in the brain white matter, including bending, fanning and beading fibers. Our source code will be made available online.
Language Models for Music Medicine Generation
Nov 13, 2024
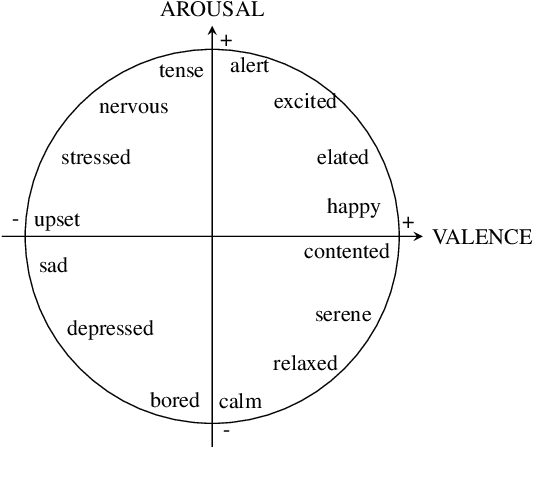
Abstract:Music therapy has been shown in recent years to provide multiple health benefits related to emotional wellness. In turn, maintaining a healthy emotional state has proven to be effective for patients undergoing treatment, such as Parkinson's patients or patients suffering from stress and anxiety. We propose fine-tuning MusicGen, a music-generating transformer model, to create short musical clips that assist patients in transitioning from negative to desired emotional states. Using low-rank decomposition fine-tuning on the MTG-Jamendo Dataset with emotion tags, we generate 30-second clips that adhere to the iso principle, guiding patients through intermediate states in the valence-arousal circumplex. The generated music is evaluated using a music emotion recognition model to ensure alignment with intended emotions. By concatenating these clips, we produce a 15-minute "music medicine" resembling a music therapy session. Our approach is the first model to leverage Language Models to generate music medicine. Ultimately, the output is intended to be used as a temporary relief between music therapy sessions with a board-certified therapist.
AFEN: Respiratory Disease Classification using Ensemble Learning
May 08, 2024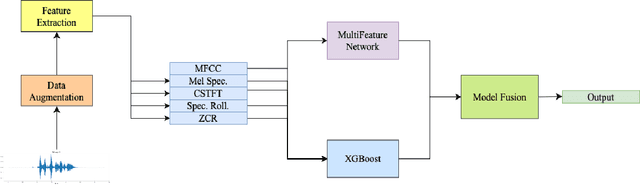
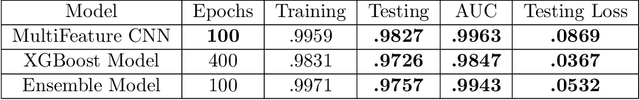
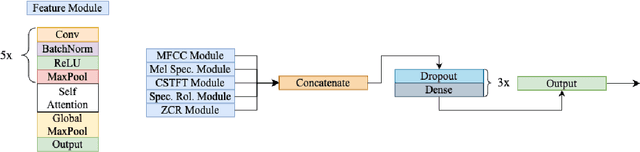
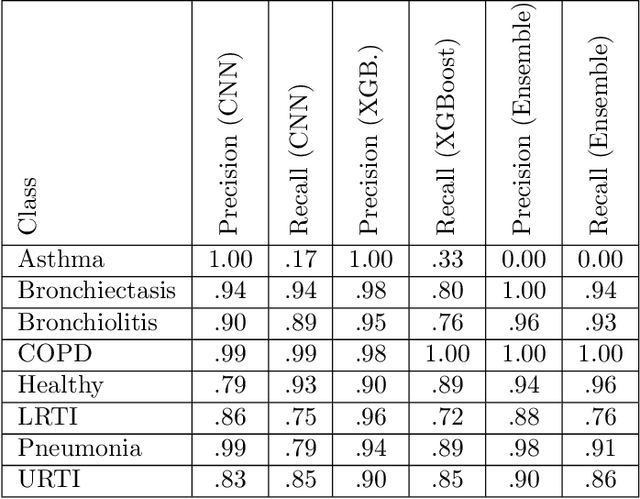
Abstract:We present AFEN (Audio Feature Ensemble Learning), a model that leverages Convolutional Neural Networks (CNN) and XGBoost in an ensemble learning fashion to perform state-of-the-art audio classification for a range of respiratory diseases. We use a meticulously selected mix of audio features which provide the salient attributes of the data and allow for accurate classification. The extracted features are then used as an input to two separate model classifiers 1) a multi-feature CNN classifier and 2) an XGBoost Classifier. The outputs of the two models are then fused with the use of soft voting. Thus, by exploiting ensemble learning, we achieve increased robustness and accuracy. We evaluate the performance of the model on a database of 920 respiratory sounds, which undergoes data augmentation techniques to increase the diversity of the data and generalizability of the model. We empirically verify that AFEN sets a new state-of-the-art using Precision and Recall as metrics, while decreasing training time by 60%.
BMapOpt: Optimization of Brain Tissue Probability Maps using a Differentiable MRI Simulator
Apr 23, 2024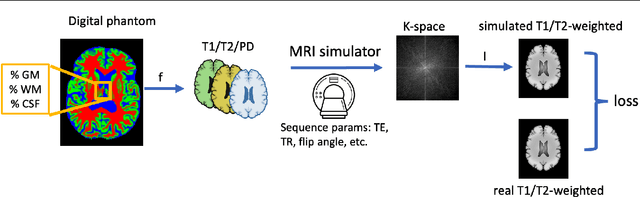
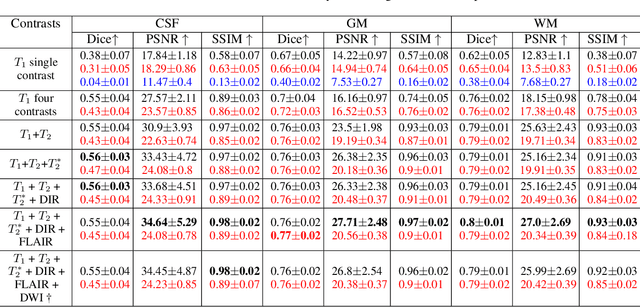
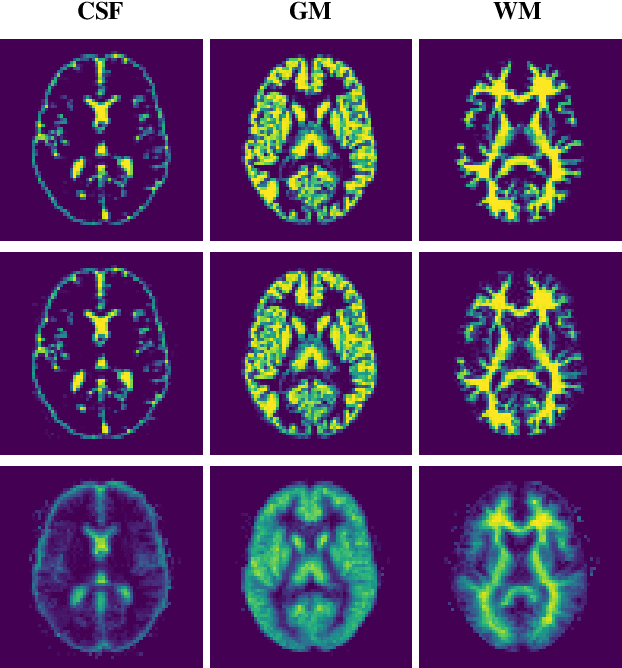
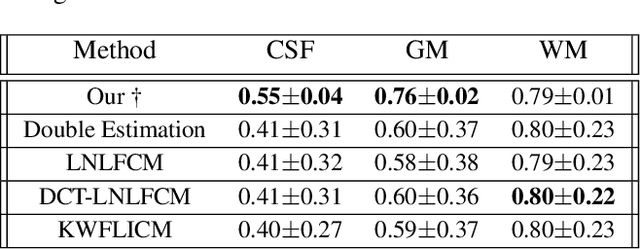
Abstract:Reconstructing digital brain phantoms in the form of multi-channeled brain tissue probability maps for individual subjects is essential for capturing brain anatomical variability, understanding neurological diseases, as well as for testing image processing methods. We demonstrate the first framework that optimizes brain tissue probability maps (Gray Matter - GM, White Matter - WM, and Cerebrospinal fluid - CSF) with the help of a Physics-based differentiable MRI simulator that models the magnetization signal at each voxel in the image. Given an observed $T_1$/$T_2$-weighted MRI scan, the corresponding clinical MRI sequence, and the MRI differentiable simulator, we optimize the simulator's input probability maps by back-propagating the L2 loss between the simulator's output and the $T_1$/$T_2$-weighted scan. This approach has the significant advantage of not relying on any training data, and instead uses the strong inductive bias of the MRI simulator. We tested the model on 20 scans from the BrainWeb database and demonstrate a highly accurate reconstruction of GM, WM, and CSF.
SQUAT: Stateful Quantization-Aware Training in Recurrent Spiking Neural Networks
Apr 15, 2024



Abstract:Weight quantization is used to deploy high-performance deep learning models on resource-limited hardware, enabling the use of low-precision integers for storage and computation. Spiking neural networks (SNNs) share the goal of enhancing efficiency, but adopt an 'event-driven' approach to reduce the power consumption of neural network inference. While extensive research has focused on weight quantization, quantization-aware training (QAT), and their application to SNNs, the precision reduction of state variables during training has been largely overlooked, potentially diminishing inference performance. This paper introduces two QAT schemes for stateful neurons: (i) a uniform quantization strategy, an established method for weight quantization, and (ii) threshold-centered quantization, which allocates exponentially more quantization levels near the firing threshold. Our results show that increasing the density of quantization levels around the firing threshold improves accuracy across several benchmark datasets. We provide an ablation analysis of the effects of weight and state quantization, both individually and combined, and how they impact models. Our comprehensive empirical evaluation includes full precision, 8-bit, 4-bit, and 2-bit quantized SNNs, using QAT, stateful QAT (SQUAT), and post-training quantization methods. The findings indicate that the combination of QAT and SQUAT enhance performance the most, but given the choice of one or the other, QAT improves performance by the larger degree. These trends are consistent all datasets. Our methods have been made available in our Python library snnTorch: https://github.com/jeshraghian/snntorch.
GaSpCT: Gaussian Splatting for Novel CT Projection View Synthesis
Apr 04, 2024



Abstract:We present GaSpCT, a novel view synthesis and 3D scene representation method used to generate novel projection views for Computer Tomography (CT) scans. We adapt the Gaussian Splatting framework to enable novel view synthesis in CT based on limited sets of 2D image projections and without the need for Structure from Motion (SfM) methodologies. Therefore, we reduce the total scanning duration and the amount of radiation dose the patient receives during the scan. We adapted the loss function to our use-case by encouraging a stronger background and foreground distinction using two sparsity promoting regularizers: a beta loss and a total variation (TV) loss. Finally, we initialize the Gaussian locations across the 3D space using a uniform prior distribution of where the brain's positioning would be expected to be within the field of view. We evaluate the performance of our model using brain CT scans from the Parkinson's Progression Markers Initiative (PPMI) dataset and demonstrate that the rendered novel views closely match the original projection views of the simulated scan, and have better performance than other implicit 3D scene representations methodologies. Furthermore, we empirically observe reduced training time compared to neural network based image synthesis for sparse-view CT image reconstruction. Finally, the memory requirements of the Gaussian Splatting representations are reduced by 17% compared to the equivalent voxel grid image representations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge