Natalia S. Rost
Hypernet-Ensemble Learning of Segmentation Probability for Medical Image Segmentation with Ambiguous Labels
Dec 13, 2021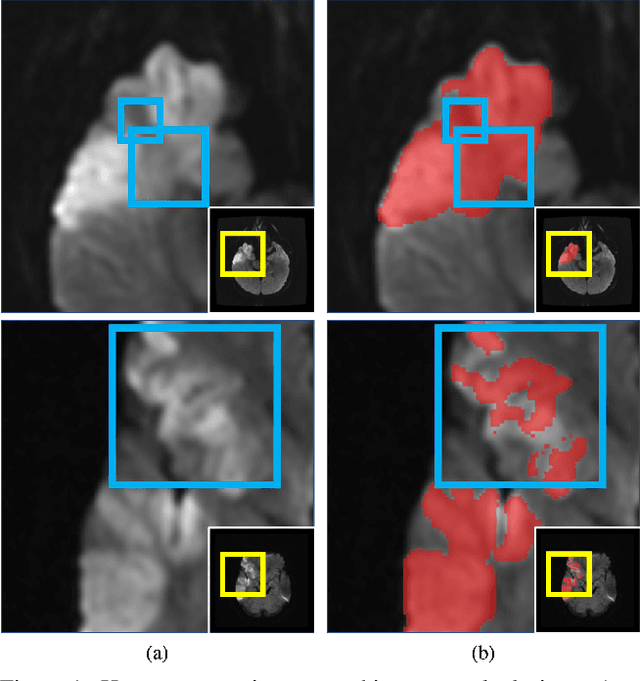
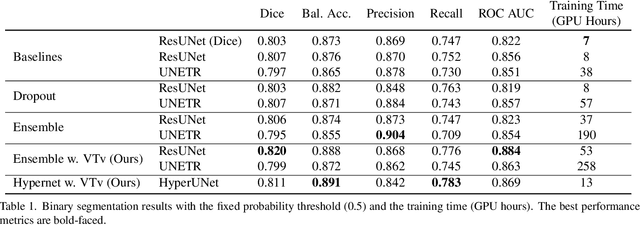
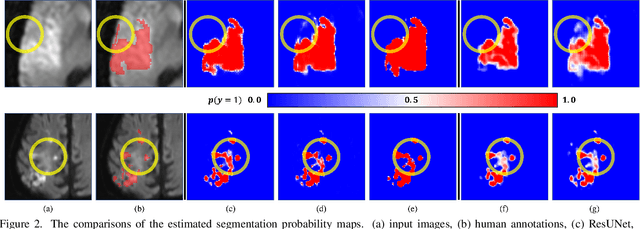
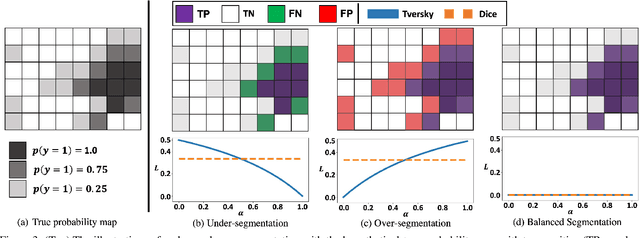
Abstract:Despite the superior performance of Deep Learning (DL) on numerous segmentation tasks, the DL-based approaches are notoriously overconfident about their prediction with highly polarized label probability. This is often not desirable for many applications with the inherent label ambiguity even in human annotations. This challenge has been addressed by leveraging multiple annotations per image and the segmentation uncertainty. However, multiple per-image annotations are often not available in a real-world application and the uncertainty does not provide full control on segmentation results to users. In this paper, we propose novel methods to improve the segmentation probability estimation without sacrificing performance in a real-world scenario that we have only one ambiguous annotation per image. We marginalize the estimated segmentation probability maps of networks that are encouraged to under-/over-segment with the varying Tversky loss without penalizing balanced segmentation. Moreover, we propose a unified hypernetwork ensemble method to alleviate the computational burden of training multiple networks. Our approaches successfully estimated the segmentation probability maps that reflected the underlying structures and provided the intuitive control on segmentation for the challenging 3D medical image segmentation. Although the main focus of our proposed methods is not to improve the binary segmentation performance, our approaches marginally outperformed the state-of-the-arts. The codes are available at \url{https://github.com/sh4174/HypernetEnsemble}.
3D-StyleGAN: A Style-Based Generative Adversarial Network for Generative Modeling of Three-Dimensional Medical Images
Jul 20, 2021
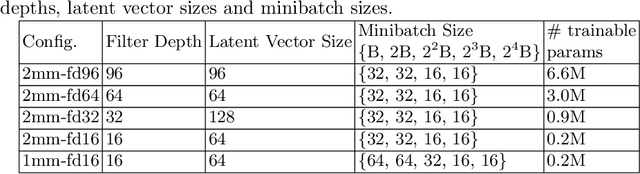
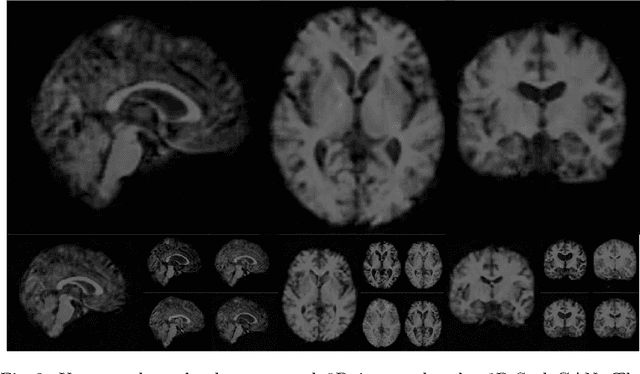

Abstract:Image synthesis via Generative Adversarial Networks (GANs) of three-dimensional (3D) medical images has great potential that can be extended to many medical applications, such as, image enhancement and disease progression modeling. However, current GAN technologies for 3D medical image synthesis need to be significantly improved to be readily adapted to real-world medical problems. In this paper, we extend the state-of-the-art StyleGAN2 model, which natively works with two-dimensional images, to enable 3D image synthesis. In addition to the image synthesis, we investigate the controllability and interpretability of the 3D-StyleGAN via style vectors inherited form the original StyleGAN2 that are highly suitable for medical applications: (i) the latent space projection and reconstruction of unseen real images, and (ii) style mixing. We demonstrate the 3D-StyleGAN's performance and feasibility with ~12,000 three-dimensional full brain MR T1 images, although it can be applied to any 3D volumetric images. Furthermore, we explore different configurations of hyperparameters to investigate potential improvement of the image synthesis with larger networks. The codes and pre-trained networks are available online: https://github.com/sh4174/3DStyleGAN.
Patient-specific Conditional Joint Models of Shape, Image Features and Clinical Indicators
Jul 17, 2019

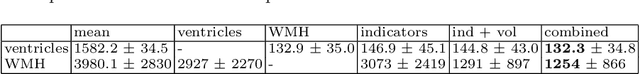
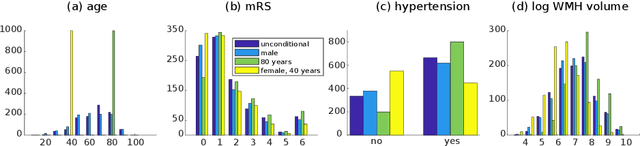
Abstract:We propose and demonstrate a joint model of anatomical shapes, image features and clinical indicators for statistical shape modeling and medical image analysis. The key idea is to employ a copula model to separate the joint dependency structure from the marginal distributions of variables of interest. This separation provides flexibility on the assumptions made during the modeling process. The proposed method can handle binary, discrete, ordinal and continuous variables. We demonstrate a simple and efficient way to include binary, discrete and ordinal variables into the modeling. We build Bayesian conditional models based on observed partial clinical indicators, features or shape based on Gaussian processes capturing the dependency structure. We apply the proposed method on a stroke dataset to jointly model the shape of the lateral ventricles, the spatial distribution of the white matter hyperintensity associated with periventricular white matter disease, and clinical indicators. The proposed method yields interpretable joint models for data exploration and patient-specific statistical shape models for medical image analysis.
* Supplementary material: https://www.youtube.com/watch?v=gPoHP_iFQIA
Automated Image Registration Quality Assessment Utilizing Deep-learning based Ventricle Extraction in Clinical Data
Jul 01, 2019

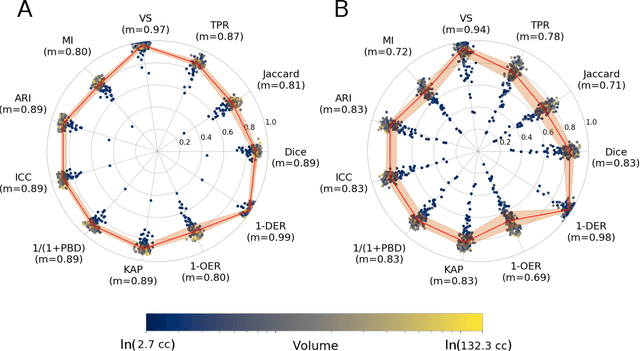
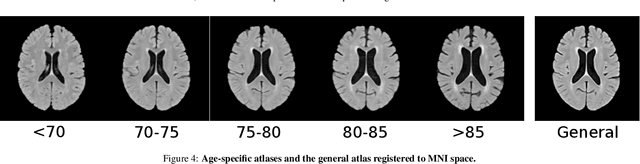
Abstract:Registration is a core component of many imaging pipelines. In case of clinical scans, with lower resolution and sometimes substantial motion artifacts, registration can produce poor results. Visual assessment of registration quality in large clinical datasets is inefficient. In this work, we propose to automatically assess the quality of registration to an atlas in clinical FLAIR MRI scans of the brain. The method consists of automatically segmenting the ventricles of a given scan using a neural network, and comparing the segmentation to the atlas' ventricles propagated to image space. We used the proposed method to improve clinical image registration to a general atlas by computing multiple registrations and then selecting the registration that yielded the highest ventricle overlap. Methods were evaluated in a single-site dataset of more than 1000 scans, as well as a multi-center dataset comprising 142 clinical scans from 12 sites. The automated ventricle segmentation reached a Dice coefficient with manual annotations of 0.89 in the single-site dataset, and 0.83 in the multi-center dataset. Registration via age-specific atlases could improve ventricle overlap compared to a direct registration to the general atlas (Dice similarity coefficient increase up to 0.15). Experiments also showed that selecting scans with the registration quality assessment method could improve the quality of average maps of white matter hyperintensity burden, instead of using all scans for the computation of the white matter hyperintensity map. In this work, we demonstrated the utility of an automated tool for assessing image registration quality in clinical scans. This image quality assessment step could ultimately assist in the translation of automated neuroimaging pipelines to the clinic.
Fast Learning-based Registration of Sparse Clinical Images
Dec 17, 2018



Abstract:Deformable registration of clinical scans is a fundamental task for many applications, such as population studies or the monitoring of long-term disease progression in individual patients. This task is challenging because, in contrast to high-resolution research-quality scans, clinical images are often sparse, missing up to 85% of the slices in comparison. Furthermore, the anatomy in the acquired slices is not consistent across scans because of variations in patient orientation with respect to the scanner. In this work, we introduce Sparse VoxelMorph (SparseVM), which adapts a state-of-the-art learning-based registration method to improve the registration of sparse clinical images. SparseVM is a fast, unsupervised method that weights voxel contributions to registration in proportion to confidence in the voxels. This leads to improved registration performance on volumes with voxels of varying reliability, such as interpolated clinical scans. SparseVM registers 3D scans in under a second on the GPU, which is orders of magnitudes faster than the best performing clinical registration methods, while still achieving comparable accuracy. Because of its short runtimes and accurate behavior, SparseVM can enable clinical analyses not previously possible. The code is publicly available at voxelmorph.mit.edu.
Medical Image Imputation from Image Collections
Aug 17, 2018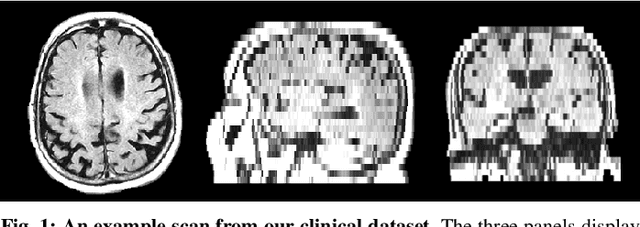

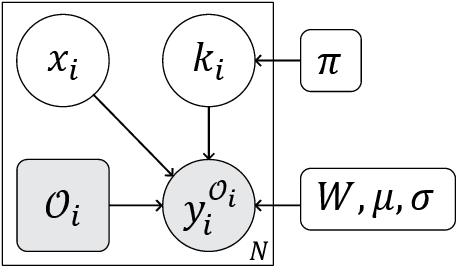

Abstract:We present an algorithm for creating high resolution anatomically plausible images consistent with acquired clinical brain MRI scans with large inter-slice spacing. Although large data sets of clinical images contain a wealth of information, time constraints during acquisition result in sparse scans that fail to capture much of the anatomy. These characteristics often render computational analysis impractical as many image analysis algorithms tend to fail when applied to such images. Highly specialized algorithms that explicitly handle sparse slice spacing do not generalize well across problem domains. In contrast, we aim to enable application of existing algorithms that were originally developed for high resolution research scans to significantly undersampled scans. We introduce a generative model that captures fine-scale anatomical structure across subjects in clinical image collections and derive an algorithm for filling in the missing data in scans with large inter-slice spacing. Our experimental results demonstrate that the resulting method outperforms state-of-the-art upsampling super-resolution techniques, and promises to facilitate subsequent analysis not previously possible with scans of this quality. Our implementation is freely available at https://github.com/adalca/papago .
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge